In this question you will use the one-dimensional particles in a box model to approximate the absorption wavelength of a cyanine dye. To determine the "box-length" for the model, determine the total length along the conjugated part of the molecule (both single and double bonds) use the following bond lengths: Typical CC bond length (both single/double bond) = 0.14 nm Typical CN bond length (both single/double bond) = 0.13 nm The structure of a cyanine dye is shown below. You need to determine the wavelength of light for a cyanine dye with n=7. Consider that both nitrogens are conjugated, so consider two CN bonds when determining box length. The left-most nitrogen has two electrons (lone pair) that count towards the number of electrons in the box, but the right-most nitrogen has no lone pair, just the double-bond. Provide your answer as the wavelength of light absorbed in units of nm. R R n R R
In this question you will use the one-dimensional particles in a box model to approximate the absorption wavelength of a cyanine dye. To determine the "box-length" for the model, determine the total length along the conjugated part of the molecule (both single and double bonds) use the following bond lengths: Typical CC bond length (both single/double bond) = 0.14 nm Typical CN bond length (both single/double bond) = 0.13 nm The structure of a cyanine dye is shown below. You need to determine the wavelength of light for a cyanine dye with n=7. Consider that both nitrogens are conjugated, so consider two CN bonds when determining box length. The left-most nitrogen has two electrons (lone pair) that count towards the number of electrons in the box, but the right-most nitrogen has no lone pair, just the double-bond. Provide your answer as the wavelength of light absorbed in units of nm. R R n R R
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter8: An Introduction To Optical Atomic Spectrometry
Section: Chapter Questions
Problem 8.12QAP
Related questions
Question
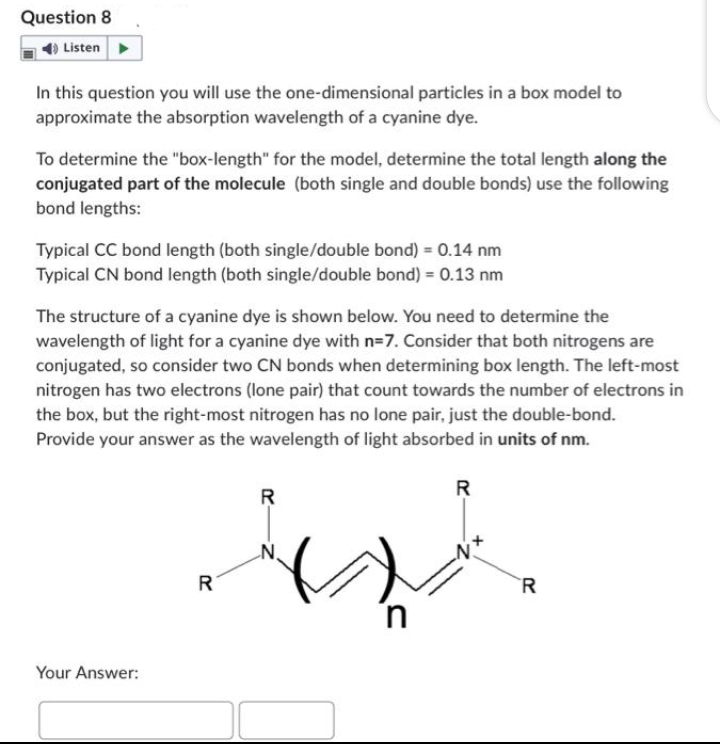
Transcribed Image Text:Question 8
Listen
In this question you will use the one-dimensional particles in a box model to
approximate the absorption wavelength of a cyanine dye.
To determine the "box-length" for the model, determine the total length along the
conjugated part of the molecule (both single and double bonds) use the following
bond lengths:
Typical CC bond length (both single/double bond) = 0.14 nm
Typical CN bond length (both single/double bond) = 0.13 nm
The structure of a cyanine dye is shown below. You need to determine the
wavelength of light for a cyanine dye with n=7. Consider that both nitrogens are
conjugated, so consider two CN bonds when determining box length. The left-most
nitrogen has two electrons (lone pair) that count towards the number of electrons in
the box, but the right-most nitrogen has no lone pair, just the double-bond.
Provide your answer as the wavelength of light absorbed in units of nm.
Your Answer:
R
R
R
R
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning