Increasing anion-to-cation ratio F Sc F Na Mg NaF MGF2 SCF3 Cation coordination number Cation coordination Octahedral Octahedral Octahedral geometry Anion coordination number 6 2 Anion coordination Octahedral Trigonal planar Linear geometry A Figure 12.27 Coordination numbers depend on stoichiometry. The sizes of the ions have been reduced to show the coordination environments clearly. Activa Go to
Increasing anion-to-cation ratio F Sc F Na Mg NaF MGF2 SCF3 Cation coordination number Cation coordination Octahedral Octahedral Octahedral geometry Anion coordination number 6 2 Anion coordination Octahedral Trigonal planar Linear geometry A Figure 12.27 Coordination numbers depend on stoichiometry. The sizes of the ions have been reduced to show the coordination environments clearly. Activa Go to
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter20: Chemistry Of Selected Transition Elements And Coordination Compounds
Section: Chapter Questions
Problem 116QRT
Related questions
Question
How many cations are there per unit cell for each of these structures?
How many anions per unit cell?
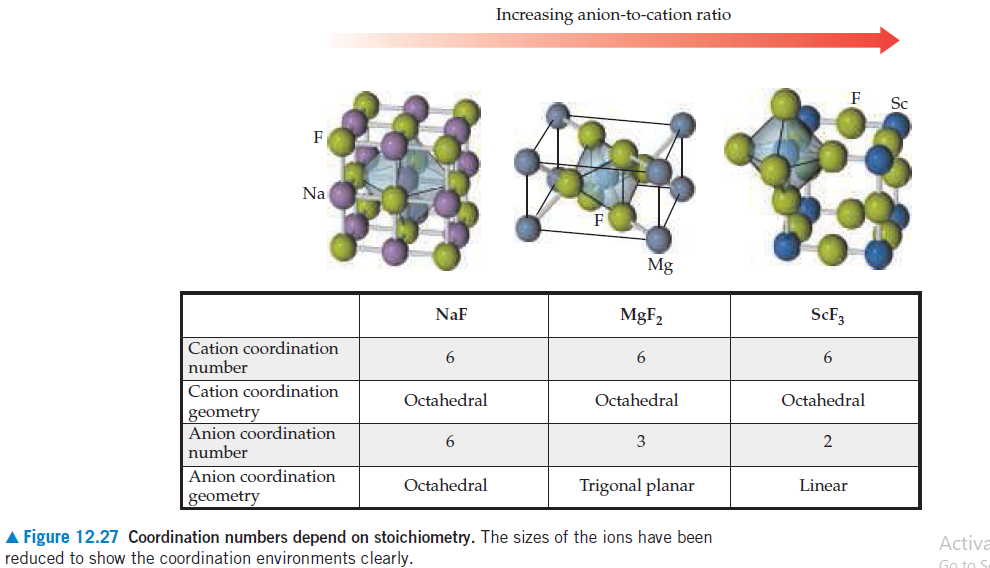
Transcribed Image Text:Increasing anion-to-cation ratio
F
Sc
F
Na
Mg
NaF
MGF2
SCF3
Cation coordination
number
Cation coordination
Octahedral
Octahedral
Octahedral
geometry
Anion coordination
number
6
2
Anion coordination
Octahedral
Trigonal planar
Linear
geometry
A Figure 12.27 Coordination numbers depend on stoichiometry. The sizes of the ions have been
reduced to show the coordination environments clearly.
Activa
Go to
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning