Iron ore is reduced to pure iron by smelting, during which the iron (III) oxide in the ore reacts with carbon monaxide gas, like this: Fe,0, ()+3C0()- 2Fe(s)+3C0, () Suppose an engineer decides to study the rate of this reaction. He prepares four reaction vessels with 110.2 g of solid iron (II) oxide and 16.3 g of carbon monoxide gas each. The volume and temperature of each vessel is shown in the table below. Arrange the reaction vessels in decreasing order of initial rate of reaction. In other words, select a "1" next to the vessel in which the engineer can reasonably expect the initial rate of reaction to be highest, a "2" next to the vessel in which the initial rate of reaction would be next highest, and so on. vessel volume temperature initial rate of reaction 2.0 L 1100. °C 4.0 L 1100. °C 2.0 L 1200. °C D 4.0 L 1000. °C
Iron ore is reduced to pure iron by smelting, during which the iron (III) oxide in the ore reacts with carbon monaxide gas, like this: Fe,0, ()+3C0()- 2Fe(s)+3C0, () Suppose an engineer decides to study the rate of this reaction. He prepares four reaction vessels with 110.2 g of solid iron (II) oxide and 16.3 g of carbon monoxide gas each. The volume and temperature of each vessel is shown in the table below. Arrange the reaction vessels in decreasing order of initial rate of reaction. In other words, select a "1" next to the vessel in which the engineer can reasonably expect the initial rate of reaction to be highest, a "2" next to the vessel in which the initial rate of reaction would be next highest, and so on. vessel volume temperature initial rate of reaction 2.0 L 1100. °C 4.0 L 1100. °C 2.0 L 1200. °C D 4.0 L 1000. °C
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter11: Chemical Kinetics
Section: Chapter Questions
Problem 11.30PAE: The rate of the decomposition of hydrogen peroxide, H2O2, depends on the concentration of iodide ion...
Related questions
Question
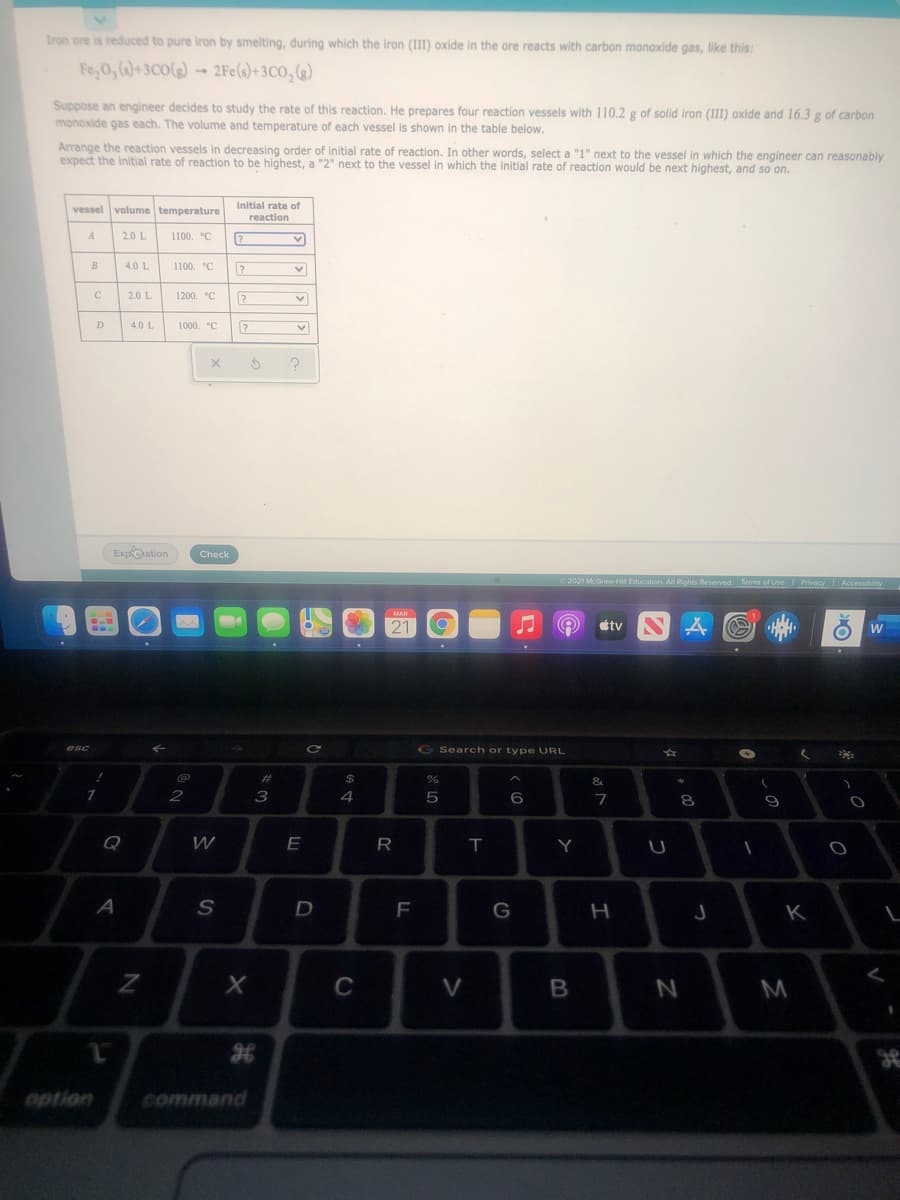
Transcribed Image Text:Iron ore is reduced to pure iron by smelting, during which the iron (III) oxide in the ore reacts with carbon monoxide gas, like this:
Fe,0, ()+3C0(g) - 2Fe(s)+3CO,(g)
Suppose an engineer decides to study the rate of this reaction. He prepares four reaction vessels with 110.2 g of solid iron (III) oxide and 16.3 g of carbon
monoxide gas each. The volume and temperature of each vessel is shown in the table below.
Arrange the reaction vessels in decreasing order of initial rate of reaction. In other words, select a "1" next to the vessel in which the engineer can reasonably
expect the initial rate of reaction to be highest, a "2" next to the vessel in which the initial rate of reaction would be next highest, and so on.
Initial rate of
vessel
volume temperature
reaction
2.0 L
1100. °C
B
4.0 L
1100. °C
C
2.0 L
1200. °C
D
4.0 L
1000. °C
Expation
Check
2021 McGraw Hi Education. Al Rights Reserved Terms of Use Pivacy Accessibiaty
21
tv
G Search or type URL
23
&
1
3
4
7
8
Q
W
E
Y
U
A
S
D
F
G
K
C
V
M
option
command
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning