is carried out isothermally in a continuous flow reactor. Calculate both the CSTR and the PFR reactor volumes (if it is possible) necessary to consurme 96% of S (ie. S = 0.04 *S) when the entering volumetric flow rate is at a constant 12 L/hr. Assume the reaction rate -s follows normal Michaelis-Menten kinetics (the product formation step is slow and rate limiting) where the substrate binds with the enzyme to form complex ES. Assume that there is no immobilization or inhibition effects present. a. So-1.5 mol/L and Vmax-4.50 mol/(hr L) and Km- 0.0300 mol/L b. S,-0.030 mol/L and Vmax = 0.10 mol/(hr*L) and Km = 0.095 mol/L c. So= 0.90 mol/L and Vmax 0.10 mol/(hr*L) and Km = 0.0750 mol/L lfitismotpossibletodetermine thereactor vol esforthedata given above, l tthe remai ingvariables that are needed to determine the reactor volumes.
is carried out isothermally in a continuous flow reactor. Calculate both the CSTR and the PFR reactor volumes (if it is possible) necessary to consurme 96% of S (ie. S = 0.04 *S) when the entering volumetric flow rate is at a constant 12 L/hr. Assume the reaction rate -s follows normal Michaelis-Menten kinetics (the product formation step is slow and rate limiting) where the substrate binds with the enzyme to form complex ES. Assume that there is no immobilization or inhibition effects present. a. So-1.5 mol/L and Vmax-4.50 mol/(hr L) and Km- 0.0300 mol/L b. S,-0.030 mol/L and Vmax = 0.10 mol/(hr*L) and Km = 0.095 mol/L c. So= 0.90 mol/L and Vmax 0.10 mol/(hr*L) and Km = 0.0750 mol/L lfitismotpossibletodetermine thereactor vol esforthedata given above, l tthe remai ingvariables that are needed to determine the reactor volumes.
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
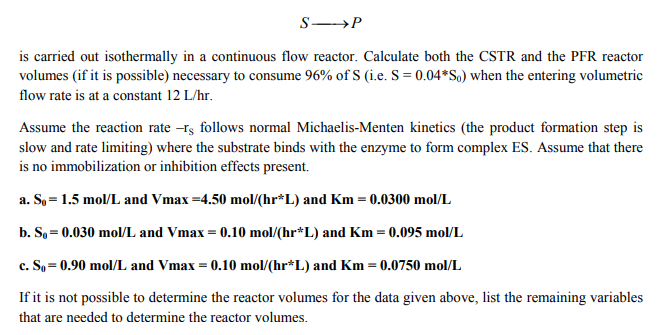
Transcribed Image Text:is carried out isothermally in a continuous flow reactor. Calculate both the CSTR and the PFR reactor
volumes (if it is possible) necessary to consurme 96% of S (ie. S = 0.04 *S) when the entering volumetric
flow rate is at a constant 12 L/hr.
Assume the reaction rate -s follows normal Michaelis-Menten kinetics (the product formation step is
slow and rate limiting) where the substrate binds with the enzyme to form complex ES. Assume that there
is no immobilization or inhibition effects present.
a. So-1.5 mol/L and Vmax-4.50 mol/(hr L) and Km- 0.0300 mol/L
b. S,-0.030 mol/L and Vmax = 0.10 mol/(hr*L) and Km = 0.095 mol/L
c. So= 0.90 mol/L and Vmax 0.10 mol/(hr*L) and Km = 0.0750 mol/L
lfitismotpossibletodetermine thereactor vol esforthedata given above, l tthe remai ingvariables
that are needed to determine the reactor volumes.
Expert Solution
Trending now
This is a popular solution!
Step by step
Solved in 10 steps with 9 images

Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The