is naturally occuring in the stratosphere where it protects the surface of the earth from the sun's UV Ozone radiation. is a pollutant formed during the combustion of coal and Nitrogen dioxide oil. is a pollutant formed when NO, formed in internal combustion engines, combines with the oxygen in the atmosphere. Sulfur dioxide is a substitute for CFC's, because it decomposes in the troposphere and therefore is not implicated Dichloromethane in the destruction of ozone in the stratosphere. Previous 6:39 PM 3/21/2020 FLV
is naturally occuring in the stratosphere where it protects the surface of the earth from the sun's UV Ozone radiation. is a pollutant formed during the combustion of coal and Nitrogen dioxide oil. is a pollutant formed when NO, formed in internal combustion engines, combines with the oxygen in the atmosphere. Sulfur dioxide is a substitute for CFC's, because it decomposes in the troposphere and therefore is not implicated Dichloromethane in the destruction of ozone in the stratosphere. Previous 6:39 PM 3/21/2020 FLV
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter5: Gases
Section: Chapter Questions
Problem 68QAP
Related questions
Question
Ozone and pollution compound:
Match the name of the substance on the left with its description on the right.
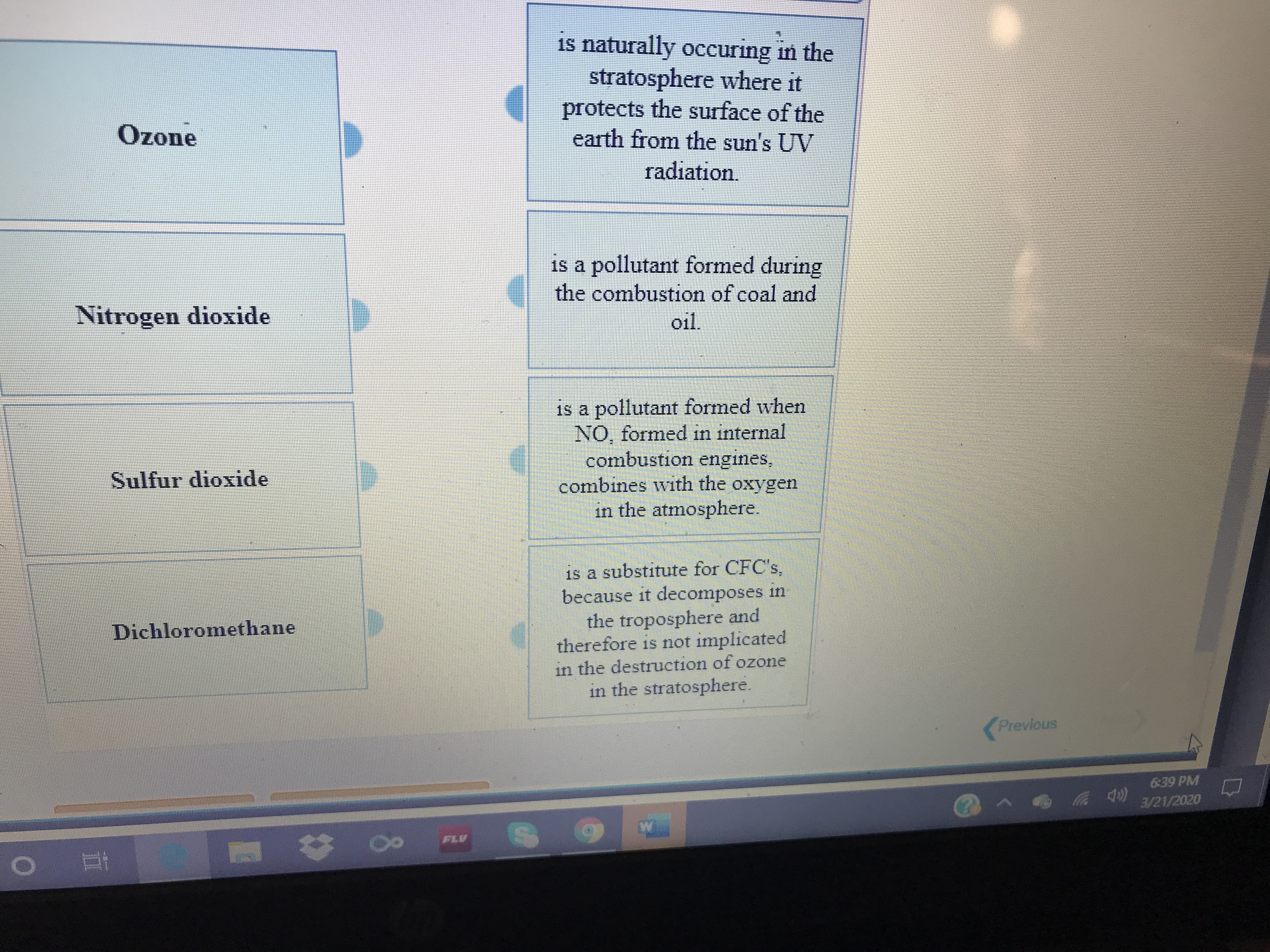
Transcribed Image Text:is naturally occuring in the
stratosphere where it
protects the surface of the
earth from the sun's UV
Ozone
radiation.
is a pollutant formed during
the combustion of coal and
Nitrogen dioxide
oil.
is a pollutant formed when
NO, formed in internal
combustion engines,
combines with the oxygen
in the atmosphere.
Sulfur dioxide
is a substitute for CFC's,
because it decomposes in
the troposphere and
therefore is not implicated
Dichloromethane
in the destruction of ozone
in the stratosphere.
Previous
6:39 PM
3/21/2020
FLV
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning