It is difficult to extinguish a fire on a crude oil tanker, because each liter of crude oil releases 2.80 x 107 J of energy when burned. To illustrate this difficulty, calculate the number of liters of water that must be expended to absorb the energy released by burning 1.00 L of crude oil, if the water has its temperature raised from 18.5 °C to 100 °C, it boils, and the resulting steam is raised to 295 °C. Use 4186 J/(kg-ºC) for the specific heat of water and 2020 J/(kg.°C) for the specific heat of steam.
It is difficult to extinguish a fire on a crude oil tanker, because each liter of crude oil releases 2.80 x 107 J of energy when burned. To illustrate this difficulty, calculate the number of liters of water that must be expended to absorb the energy released by burning 1.00 L of crude oil, if the water has its temperature raised from 18.5 °C to 100 °C, it boils, and the resulting steam is raised to 295 °C. Use 4186 J/(kg-ºC) for the specific heat of water and 2020 J/(kg.°C) for the specific heat of steam.
Related questions
Question
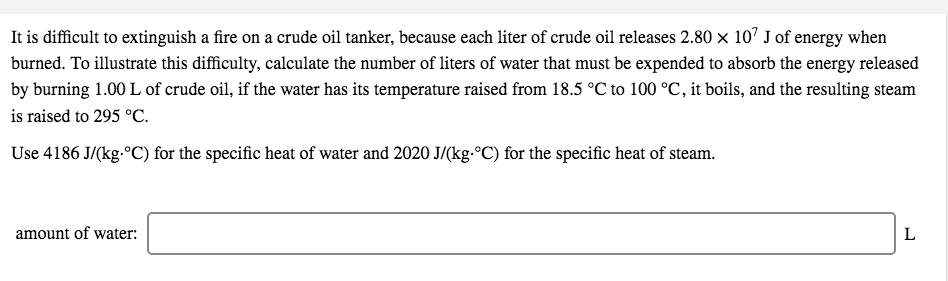
Transcribed Image Text:It is difficult to extinguish a fire on a crude oil tanker, because each liter of crude oil releases 2.80 x 107 J of energy when
burned. To illustrate this difficulty, calculate the number of liters of water that must be expended to absorb the energy released
by burning 1.00 L of crude oil, if the water has its temperature raised from 18.5 °C to 100 °C, it boils, and the resulting steam
is raised to 295 °C.
Use 4186 J/(kg-ºC) for the specific heat of water and 2020 J/(kg.°C) for the specific heat of steam.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images
