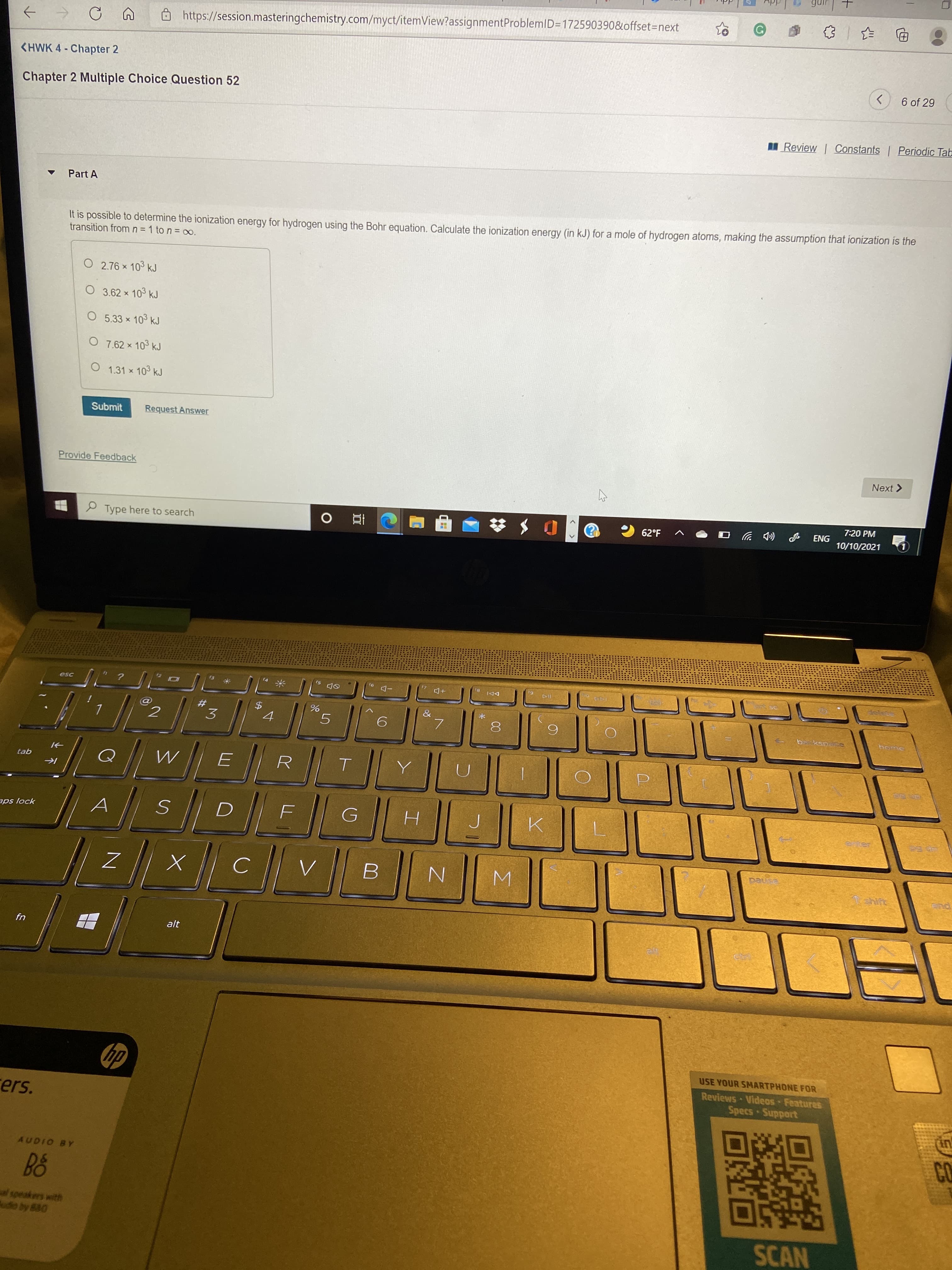
It is possible to determine the ionization energy for hydrogen using the Bohr equation. Calculate the ionization energy (in kJ) for a mole of hydrogen atoms, making the assumption that ionization is the transition from n = 1 to n = 00(infinity
It is possible to determine the ionization energy for hydrogen using the Bohr equation. Calculate the ionization energy (in kJ) for a mole of hydrogen atoms, making the assumption that ionization is the transition from n = 1 to n = 00(infinity
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter33: Automated Methods Of Analysis
Section: Chapter Questions
Problem 33.6QAP
Related questions
Question
It is possible to determine the ionization energy for hydrogen using the Bohr equation. Calculate the ionization energy (in kJ) for a mole of hydrogen atoms, making the assumption that ionization is the
transition from n = 1 to n = 00(infinity)
Transcribed Image Text:Σ
https://session.masteringchemistry.com/myct/itemView?assignmentProblemID=172590390&offset=Dnext
|企
->
<HWK 4 - Chapter 2
Chapter 2 Multiple Choice Question 52
6 of 29
Review Constants Periodic Tab
Part A
It is possible to determine the ionization energy for hydrogen using the Bohr equation. Calculate the ionization energy (in kJ) for a mole of hydrogen atoms, making the assumption that ionization is the
transition from n= 1 to n = oo.
O 2.76 x 103 kJ
O 3.62 × 103 kJ
O 5.33 x 103 kJ
O 7.62 × 103 kJ
O 1.31 x 103 kJ
Submit
Request Answer
Provide Feedback
Next >
Type here to search
7:20 PM
62°F
ENG
直。
10/10/2021
91
61
DDI
#3
3
24
4.
7.
aps lock
K.
sned
shift
alt
USE YOUR SMARTPHONE FOR
Reviews VideOs Features
ers.
Specs Support
98
SCAN
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning
