Justify your answer with calculations of partial charges on these amino acid side chains at pH = 7.8. (Hint: Consider lessons from Coulomb's law, and the Henderson-Hasselbalch equation.) Express your answers using two significant figures separated by a comma. Π| ΑΣΦ - 0.99,0.04 Submit Previous Answers Request Answer ? Incorrect: Try Again: 3 attempts remaining
Justify your answer with calculations of partial charges on these amino acid side chains at pH = 7.8. (Hint: Consider lessons from Coulomb's law, and the Henderson-Hasselbalch equation.) Express your answers using two significant figures separated by a comma. Π| ΑΣΦ - 0.99,0.04 Submit Previous Answers Request Answer ? Incorrect: Try Again: 3 attempts remaining
Chapter14: Principles Of Neutralization Titrations
Section: Chapter Questions
Problem 14.32QAP
Related questions
Question
answer part C/last question only. box the answer and MAKE SURE IT IS CORRECT
![Justify your answer with calculations of partial charges on these amino acid side chains at pH = 7.8. (Hint: Consider lessons from Coulomb's law, and the Henderson-Hasselbalch equation.)
Express your answers using two significant figures separated by a comma.
ΑΣΦ
- 0.99.0.04
Submit
www.
]] ?
Previous Answers Request Answer
Incorrect: Try Again: 3 attempts remaining](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F0ba7a132-7931-4407-922e-a5b40e804180%2F9f8f849f-ad97-411e-b330-af3de1eaf00f%2Faocolz_processed.png&w=3840&q=75)
Transcribed Image Text:Justify your answer with calculations of partial charges on these amino acid side chains at pH = 7.8. (Hint: Consider lessons from Coulomb's law, and the Henderson-Hasselbalch equation.)
Express your answers using two significant figures separated by a comma.
ΑΣΦ
- 0.99.0.04
Submit
www.
]] ?
Previous Answers Request Answer
Incorrect: Try Again: 3 attempts remaining
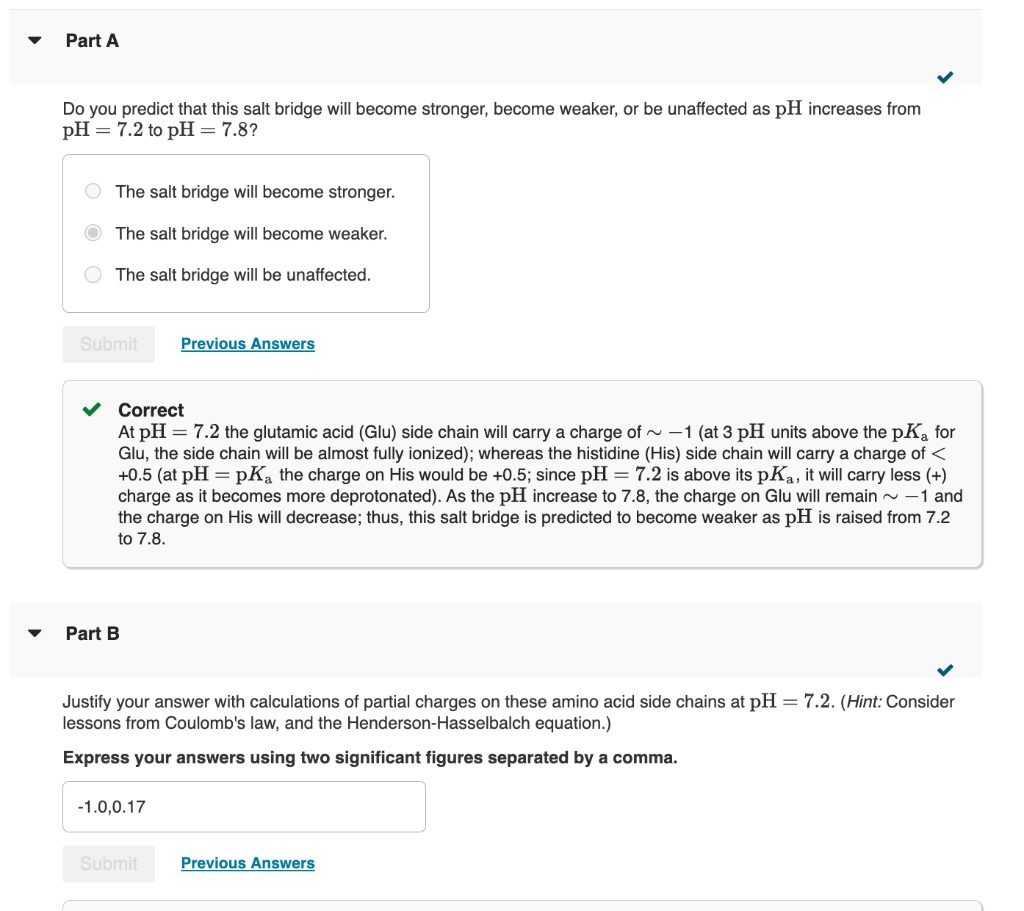
Transcribed Image Text:Part A
Do you predict that this salt bridge will become stronger, become weaker, or be unaffected as pH increases from
pH = 7.2 to pH = 7.8?
O The salt bridge will become stronger.
The salt bridge will become weaker.
The salt bridge will be unaffected.
Submit
✓ Correct
At pH = 7.2 the glutamic acid (Glu) side chain will carry a charge of ~ -1 (at 3 pH units above the pK₂ for
Glu, the side chain will be almost fully ionized); whereas the histidine (His) side chain will carry a charge of <
+0.5 (at pH=pKa the charge on His would be +0.5; since pH = 7.2 is above its pKa, it will carry less (+)
charge as it becomes more deprotonated). As the pH increase to 7.8, the charge on Glu will remain ~ -1 and
the charge on His will decrease; thus, this salt bridge is predicted to become weaker as pH is raised from 7.2
to 7.8.
Part B
Previous Answers
Justify your answer with calculations of partial charges on these amino acid side chains at pH = 7.2. (Hint: Consider
lessons from Coulomb's law, and the Henderson-Hasselbalch equation.)
Express your answers using two significant figures separated by a comma.
-1.0,0.17
Submit
Previous Answers
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning