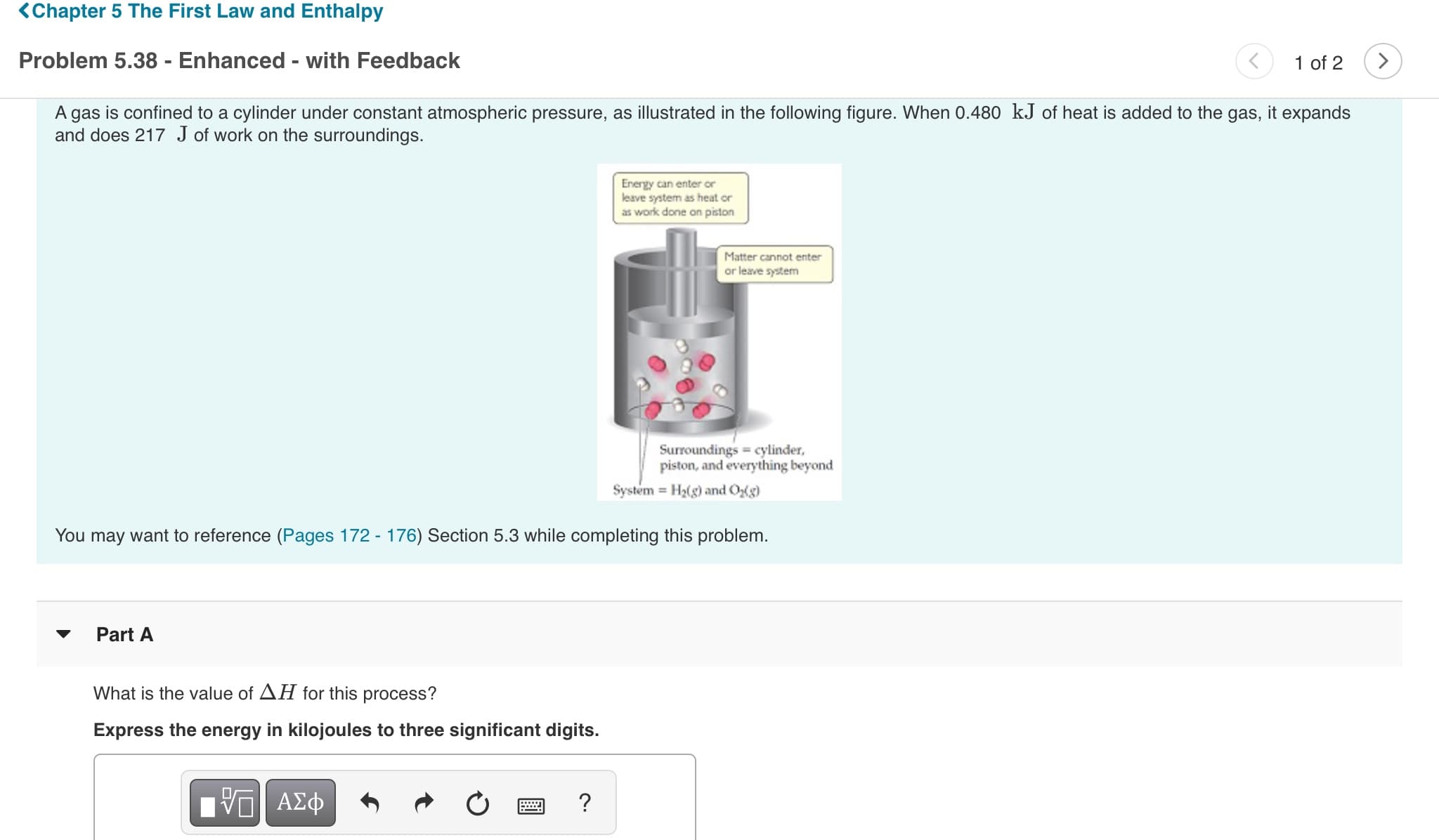
KChapter 5 The First Law and Enthalpy Problem 5.38 - Enhanced - with Feedback 1 of 2 A gas is confined to a cylinder under constant atmospheric pressure, as illustrated in the following figure. When 0.480 kJ of heat is added to the gas, it expands and does 217 J of work on the surroundings. Energy can enter or leave system as heat or as work done on piston Matter cannot enter or leave system Surroundings cylinder, piston, and everything beyond System H2(g) and Og) You may want to reference (Pages 172 - 176) Section 5.3 while completing this problem. Part A What is the value of AH for this process? Express the energy in kilojoules to three significant digits. Vα ΑΣφ plem 5.38 - Enhanced - with Feedback 1 of 2 Surroundings cylinder, piston, and everything beyond System H2(g) and Og) You may want to reference (Pages 172 - 176) Section 5.3 while completing this problem. Part A Part B What is the value of AE for this process? Express the energy in kilojoules to three decimal places. ΑΣφ ? ΔΕΞ kJ Previous Answers Request Answer Submit
KChapter 5 The First Law and Enthalpy Problem 5.38 - Enhanced - with Feedback 1 of 2 A gas is confined to a cylinder under constant atmospheric pressure, as illustrated in the following figure. When 0.480 kJ of heat is added to the gas, it expands and does 217 J of work on the surroundings. Energy can enter or leave system as heat or as work done on piston Matter cannot enter or leave system Surroundings cylinder, piston, and everything beyond System H2(g) and Og) You may want to reference (Pages 172 - 176) Section 5.3 while completing this problem. Part A What is the value of AH for this process? Express the energy in kilojoules to three significant digits. Vα ΑΣφ plem 5.38 - Enhanced - with Feedback 1 of 2 Surroundings cylinder, piston, and everything beyond System H2(g) and Og) You may want to reference (Pages 172 - 176) Section 5.3 while completing this problem. Part A Part B What is the value of AE for this process? Express the energy in kilojoules to three decimal places. ΑΣφ ? ΔΕΞ kJ Previous Answers Request Answer Submit
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter5: Principles Of Chemical Reactivity: Energy And Chemical Reactions
Section5.4: The First Law Of Thermodynamics
Problem 5.5CYU: Nitrogen gas (2.75 L) is confined in a cylinder under constant atmospheric pressure (1.01 105...
Related questions
Question
I need help on part B. Another person answered that it was .480, but it says it’s incorrect.
Transcribed Image Text:KChapter 5 The First Law and Enthalpy
Problem 5.38 - Enhanced - with Feedback
1 of 2
A gas is confined to a cylinder under constant atmospheric pressure, as illustrated in the following figure. When 0.480 kJ of heat is added to the gas, it expands
and does 217 J of work on the surroundings.
Energy can enter or
leave system as heat or
as work done on piston
Matter cannot enter
or leave system
Surroundings cylinder,
piston, and everything beyond
System H2(g) and Og)
You may want to reference (Pages 172 - 176) Section 5.3 while completing this problem.
Part A
What is the value of AH for this process?
Express the energy in kilojoules to three significant digits.
Vα ΑΣφ
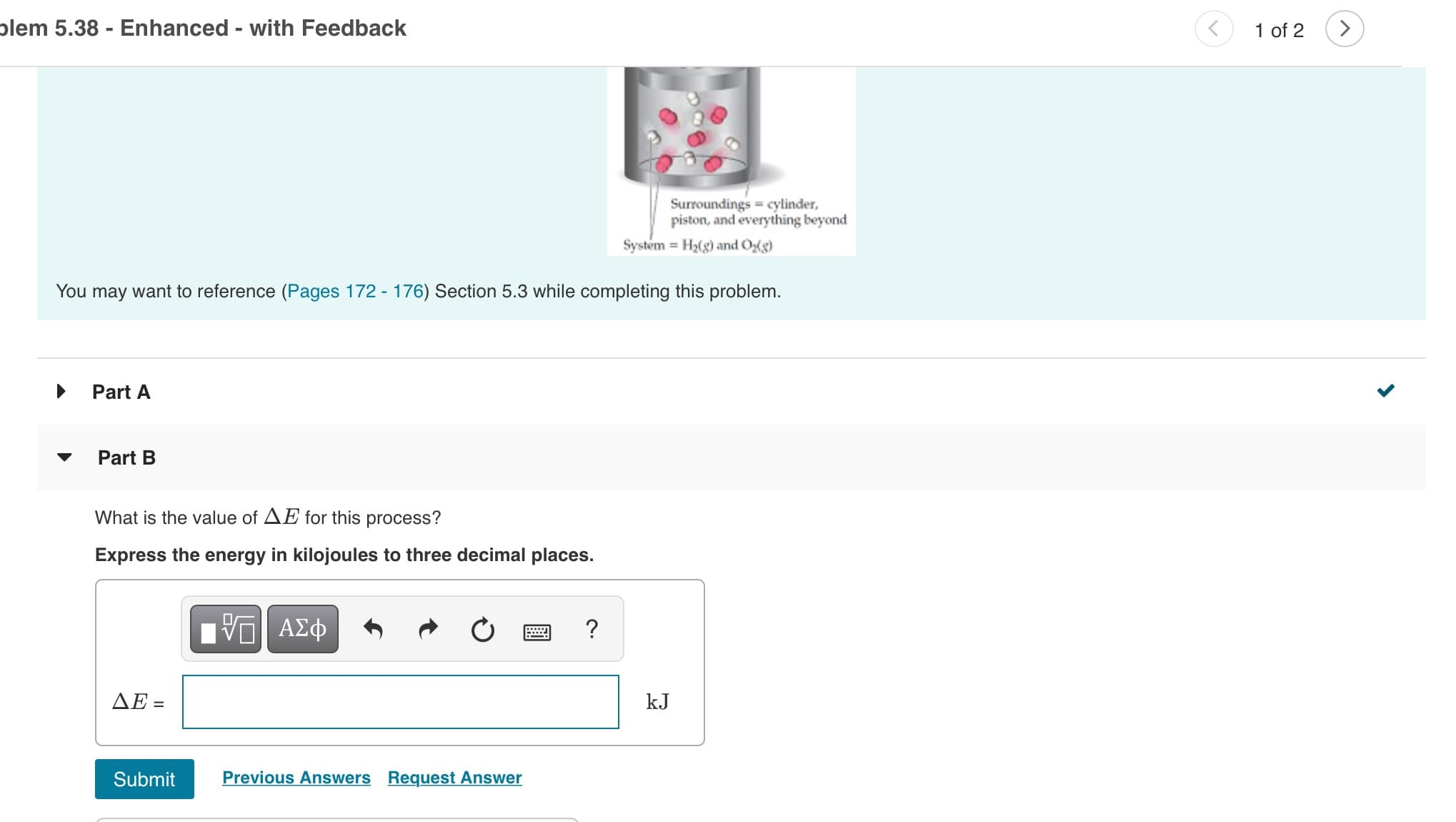
Transcribed Image Text:plem 5.38 - Enhanced - with Feedback
1 of 2
Surroundings cylinder,
piston, and everything beyond
System H2(g) and Og)
You may want to reference (Pages 172 - 176) Section 5.3 while completing this problem.
Part A
Part B
What is the value of AE for this process?
Express the energy in kilojoules to three decimal places.
ΑΣφ
?
ΔΕΞ
kJ
Previous Answers Request Answer
Submit
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning