le of commercial perchloric acid is 70.0% HCIO4 by mass, its density is 1.664 g/mL. How many ated HClo, would be required to prepare 500 ml of 1.50 МНО 104 solution? illiliters of this concentr a. 33.0 mL b. 45.3 mL c. 54.1 mL d. 64.7 mL e. 78.6 mL
le of commercial perchloric acid is 70.0% HCIO4 by mass, its density is 1.664 g/mL. How many ated HClo, would be required to prepare 500 ml of 1.50 МНО 104 solution? illiliters of this concentr a. 33.0 mL b. 45.3 mL c. 54.1 mL d. 64.7 mL e. 78.6 mL
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter15: Solutions
Section: Chapter Questions
Problem 100AP
Related questions
Question
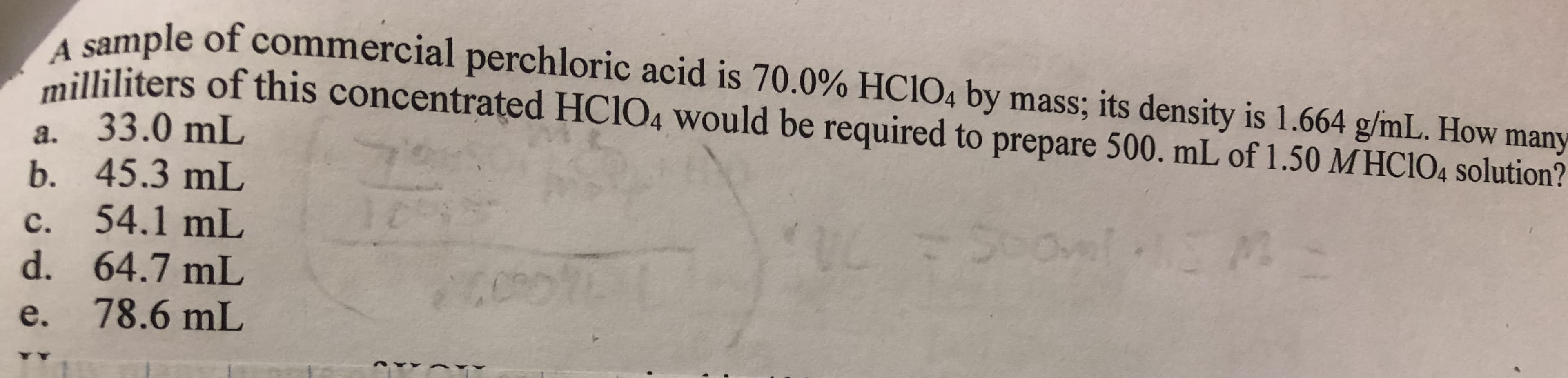
Transcribed Image Text:le of commercial perchloric acid is 70.0% HCIO4 by mass, its density is 1.664 g/mL. How many
ated HClo, would be required to prepare 500 ml of 1.50 МНО 104 solution?
illiliters of this concentr
a. 33.0 mL
b. 45.3 mL
c. 54.1 mL
d. 64.7 mL
e. 78.6 mL
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 4 images

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning