liquid density dimethyl sulfoxide -1 1.1 g-mL tetrahydrofuran -1 0.89 g mL diethylamine -1 0.71 g-mL ethanolamine 1.0 g-mL -1 0.79 g mL acetone Next, the chemist measures the volume of therunknown liquid as 1019. cm and the mass of the unknown liquid as 1.29 kg. Calculate the density of the liquid. Be sure your answer has the correct number of significant digits. g mL Given the data above, is it possible to identify the liquid? O yes O no O dimethyl sulfoxide O tetrahydrofuran O diethylamine O ethanolamine O acetone If it is possible to identify the liquid, do so.
liquid density dimethyl sulfoxide -1 1.1 g-mL tetrahydrofuran -1 0.89 g mL diethylamine -1 0.71 g-mL ethanolamine 1.0 g-mL -1 0.79 g mL acetone Next, the chemist measures the volume of therunknown liquid as 1019. cm and the mass of the unknown liquid as 1.29 kg. Calculate the density of the liquid. Be sure your answer has the correct number of significant digits. g mL Given the data above, is it possible to identify the liquid? O yes O no O dimethyl sulfoxide O tetrahydrofuran O diethylamine O ethanolamine O acetone If it is possible to identify the liquid, do so.
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter1: Chemistry And Measurement
Section: Chapter Questions
Problem 1.128QP: Providing no reaction occurs, a solid will float on any liquid that is more dense than it is. The...
Related questions
Question
100%
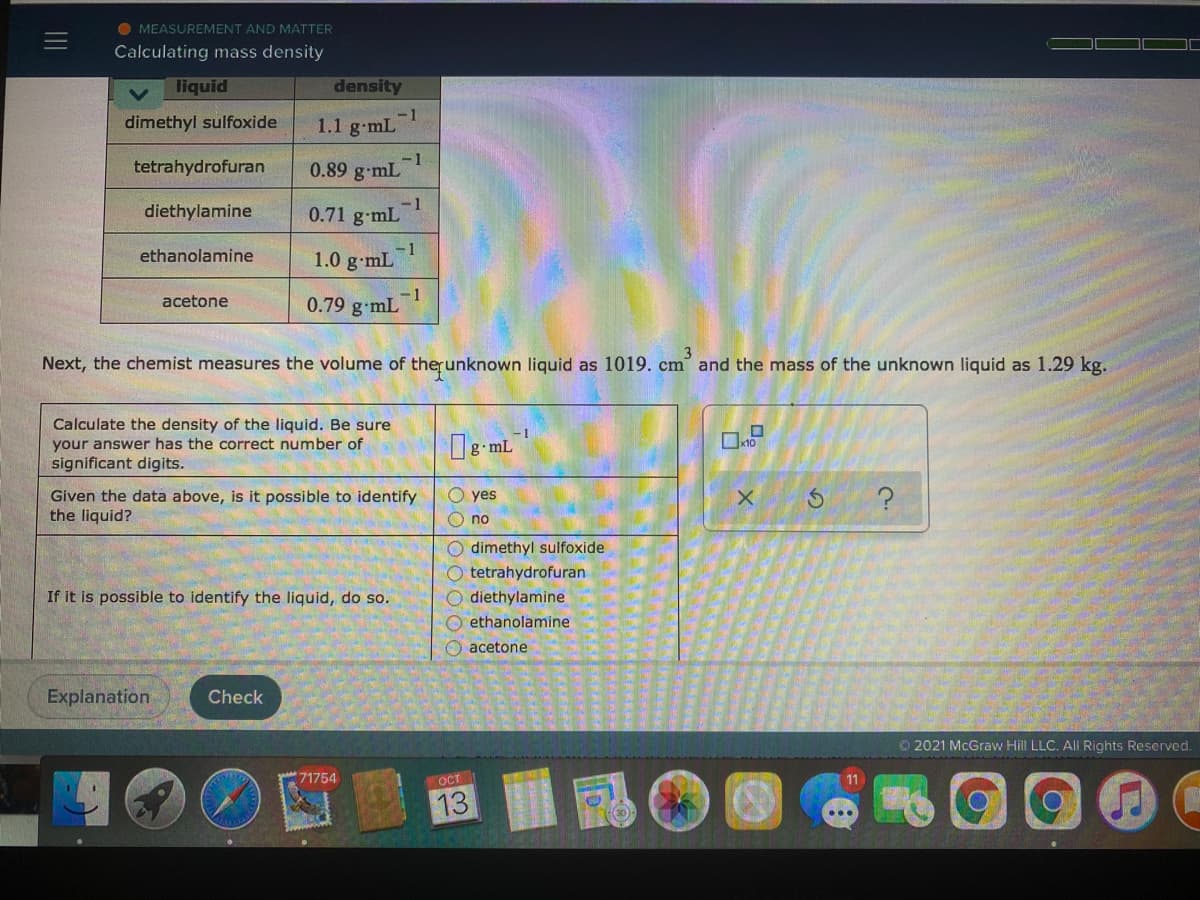
Transcribed Image Text:O MEASUREMENT AND MATTER
Calculating mass density
liquid
density
dimethyl sulfoxide
-1
1.1 g-mL
tetrahydrofuran
0.89 g-mL
diethylamine
-1
0.71 g-mL
ethanolamine
1.0 g-mL
-1
0.79 g-mL
acetone
Next, the chemist measures the volume of therunknown liquid as 1019. cm and the mass of the unknown liquid as 1.29 kg.
Calculate the density of the liquid. Be sure
your answer has the correct number of
x10
significant digits.
Given the data above, is it possible to identify
the liquid?
yes
O no
O dimethyl sulfoxide
O tetrahydrofuran
O diethylamine
O ethanolamine
If it is possible to identify the liquid, do so.
O acetone
Explanation
Check
O 2021 McGraw Hill LLC. All Rights Reserved.
71754
OCT
11
13
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER