Liquid hexane (CH3(CH, CH,) reacts with gaseous oxygen gas (02 to produce gaseous carbon dioxide (CO,) and gaseous water (H,O). If 0.852 g of water is produced from the reaction of 1.7 g of hexane and 2.6 g of oxygen gas, calculate the percent yield of water. Round your answer to 2 significant figures.
Liquid hexane (CH3(CH, CH,) reacts with gaseous oxygen gas (02 to produce gaseous carbon dioxide (CO,) and gaseous water (H,O). If 0.852 g of water is produced from the reaction of 1.7 g of hexane and 2.6 g of oxygen gas, calculate the percent yield of water. Round your answer to 2 significant figures.
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter3: Stoichiometry
Section: Chapter Questions
Problem 114E: a. Write die balanced equation for the combustion of isooctane (C8H18) to produce water vapor and...
Related questions
Question
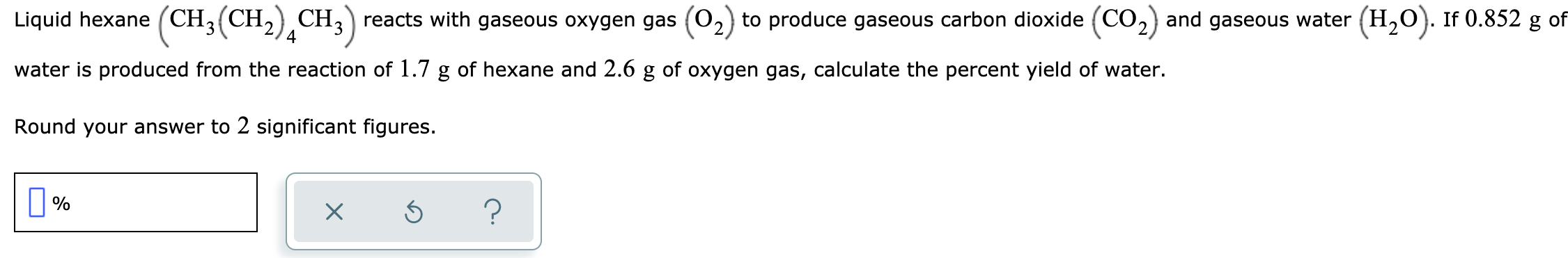
Transcribed Image Text:Liquid hexane (CH3(CH, CH,) reacts with gaseous oxygen gas (02
to produce gaseous carbon dioxide (CO,) and gaseous water (H,O). If 0.852 g of
water is produced from the reaction of 1.7 g of hexane and 2.6 g of oxygen gas, calculate the percent yield of water.
Round your answer to 2 significant figures.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 4 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning