Ma ASapling Learning A solution of a specific vitamin has Amax = 283 nm and a concentration of 7.16 x 107 M. The absorbance of th solution at 283 nm is A = 0.150. What is the molar absorptivity of the vitamin at 283 nm? (sample pathlength 1.00 cm) Number -1 L mol cm 5. Exit Next Check Answer Give Up & View Solution Previous Hint reb search
Ma ASapling Learning A solution of a specific vitamin has Amax = 283 nm and a concentration of 7.16 x 107 M. The absorbance of th solution at 283 nm is A = 0.150. What is the molar absorptivity of the vitamin at 283 nm? (sample pathlength 1.00 cm) Number -1 L mol cm 5. Exit Next Check Answer Give Up & View Solution Previous Hint reb search
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter15: Molecular Luminescence Spectrometry
Section: Chapter Questions
Problem 15.10QAP
Related questions
Question
Transcribed Image Text:Ma
ASapling Learning
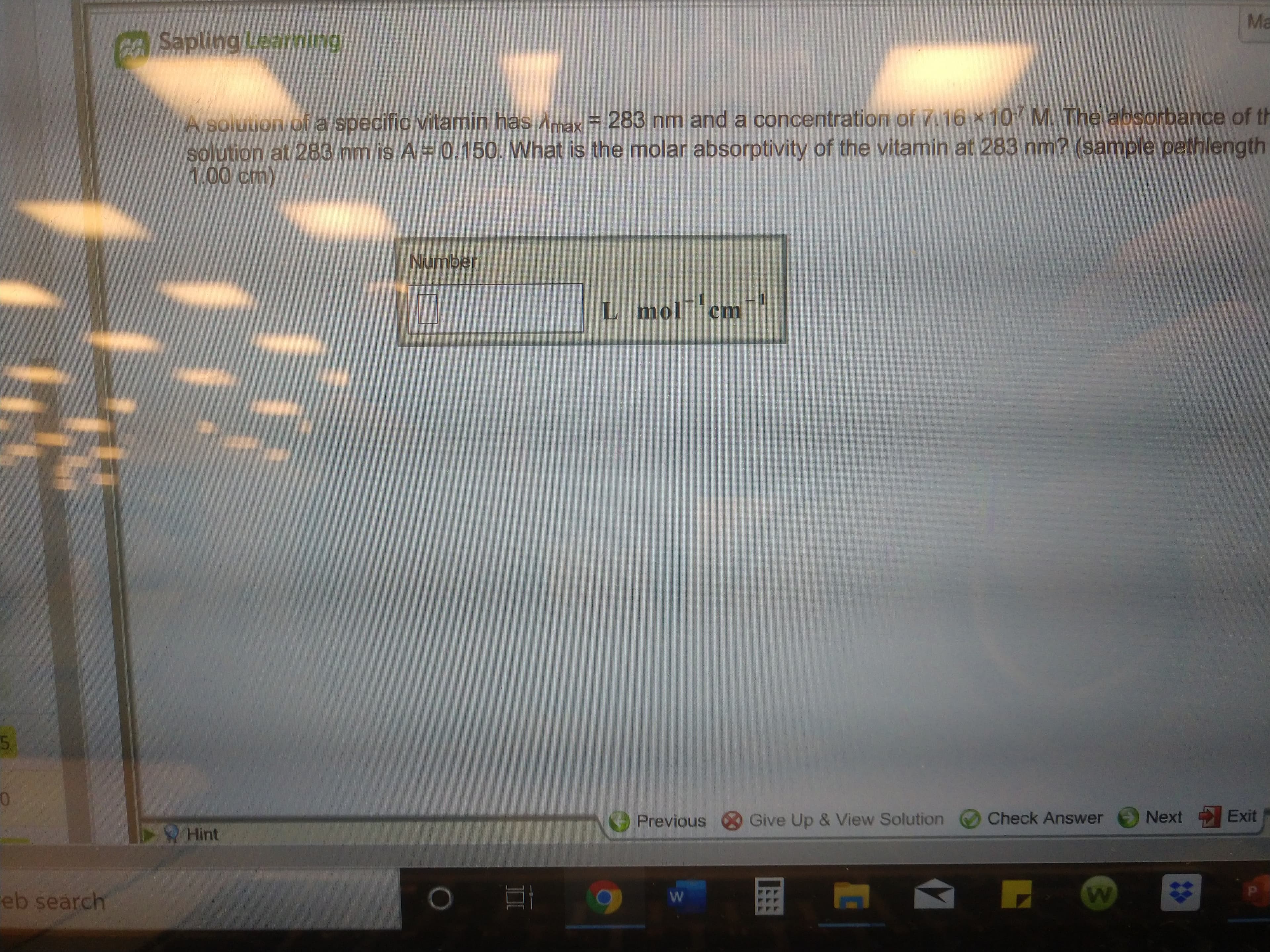
A solution of a specific vitamin has Amax = 283 nm and a concentration of 7.16 x 107 M. The absorbance of th
solution at 283 nm is A = 0.150. What is the molar absorptivity of the vitamin at 283 nm? (sample pathlength
1.00 cm)
Number
-1
L mol cm
5.
Exit
Next
Check Answer
Give Up & View Solution
Previous
Hint
reb search
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning