mass of unknown crude for acid and neutral is 0.03 grams mass of unknown recrystallized for acid and neutral is 0.03 grams. I need help in finding overall percent
mass of unknown crude for acid and neutral is 0.03 grams mass of unknown recrystallized for acid and neutral is 0.03 grams. I need help in finding overall percent
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
mass of unknown crude for acid and neutral is 0.03 grams mass of unknown recrystallized for acid and neutral is 0.03 grams. I need help in finding overall percent
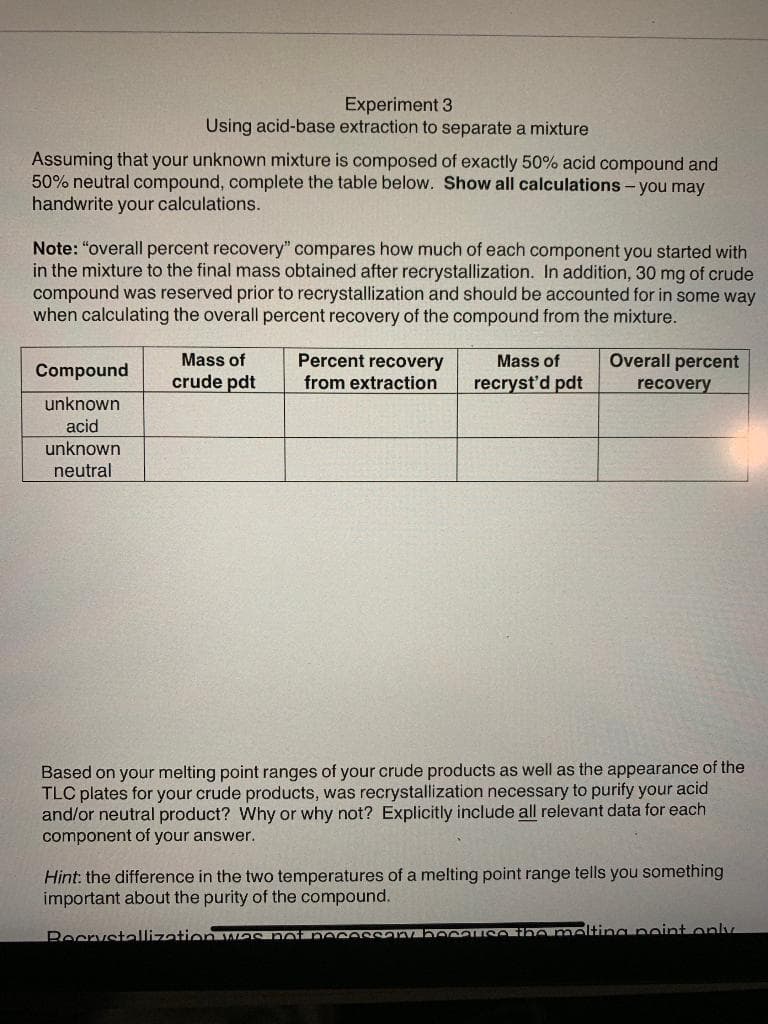
Transcribed Image Text:Experiment 3
Using acid-base extraction to separate a mixture
Assuming that your unknown mixture is composed of exactly 50% acid compound and
50% neutral compound, complete the table below. Show all calculations - you may
handwrite your calculations.
Note: "overall percent recovery" compares how much of each component you started with
in the mixture to the final mass obtained after recrystallization. In addition, 30 mg of crude
compound was reserved prior to recrystallization and should be accounted for in some way
when calculating the overall percent recovery of the compound from the mixture.
Mass of
Percent recovery
from extraction
Overall percent
Mass of
Compound
crude pdt
recryst'd pdt
recovery
unknown
acid
unknown
neutral
Based on your melting point ranges of your crude products as well as the appearance of the
TLC plates for your crude products, was recrystallization necessary to purify your acid
and/or neutral product? Why or why not? Explicitly include all relevant data for each
component of your answer.
Hint: the difference in the two temperatures of a melting point range tells you something
important about the purity of the compound.
BecrystallizationYG no eceSSoV c SO Onmelting peint only
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY