Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.9QAP
Related questions
Question
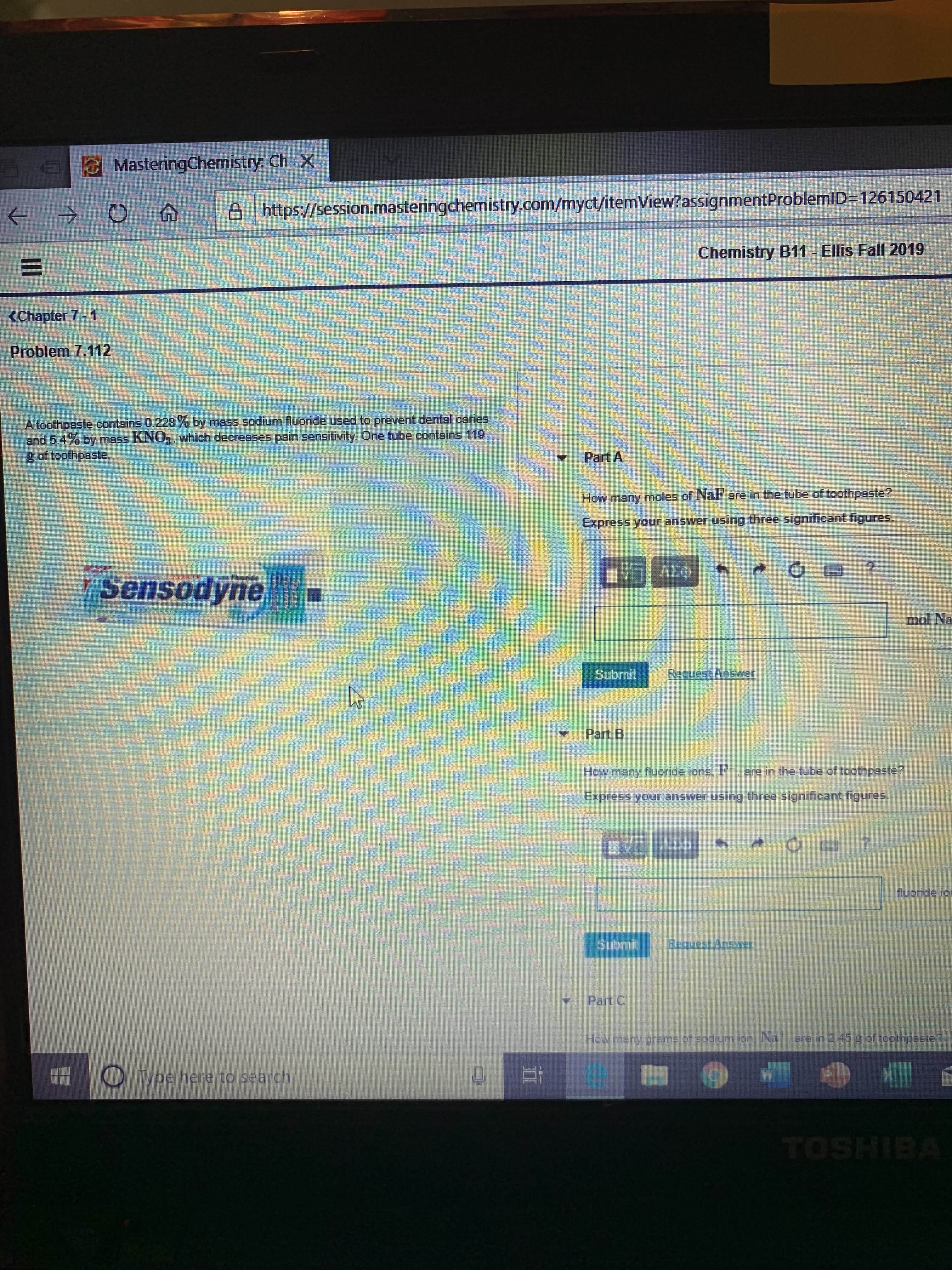
Transcribed Image Text:MasteringChemistry: Ch X
https://session.masteringchemistry.com/myct/itemView?assignmentProblemID-126150421
Ellis Fall 2019
Chemistry B11
<Chapter 7 - 1
Problem 7.112
A toothpaste contains 0.228y by mass sodium fluoride used to prevent dental caries
and 5.4% by mass KNO,, which decreases pain sensitivity. One tube contains 119
g of toothpaste.
Part A
How many moles of Nal are in the tube of toathpaste?
Express your answer using three significant figures.
AEO
Sensodyne
mol Na
Submit
Request Answer
Part B
are in the tube of toothpaste?
How many flucoride ions, l
Express your answer using three significant figures.
AX
fluoride ic
Subrnit
BaquestAnsat
Part C
Hew many grams of sodium ion, Na. 2re in 2 45 4 of toothpaste?
e
Type here to search
TOSHIBA
II
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 2 images

Recommended textbooks for you

