MasteringChemistry: Chapter X P Introduction: Chemical Quantit X + C https://session.masteringchemistry.com/myct/itemView?assignmentProblemID=114908580 Spring 2019
MasteringChemistry: Chapter X P Introduction: Chemical Quantit X + C https://session.masteringchemistry.com/myct/itemView?assignmentProblemID=114908580 Spring 2019
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.9QAP
Related questions
Question
Transcribed Image Text:MasteringChemistry: Chapter X
P Introduction: Chemical Quantit X
+
C
https://session.masteringchemistry.com/myct/itemView?assignmentProblemID=114908580
Spring 2019
<Chapter 7
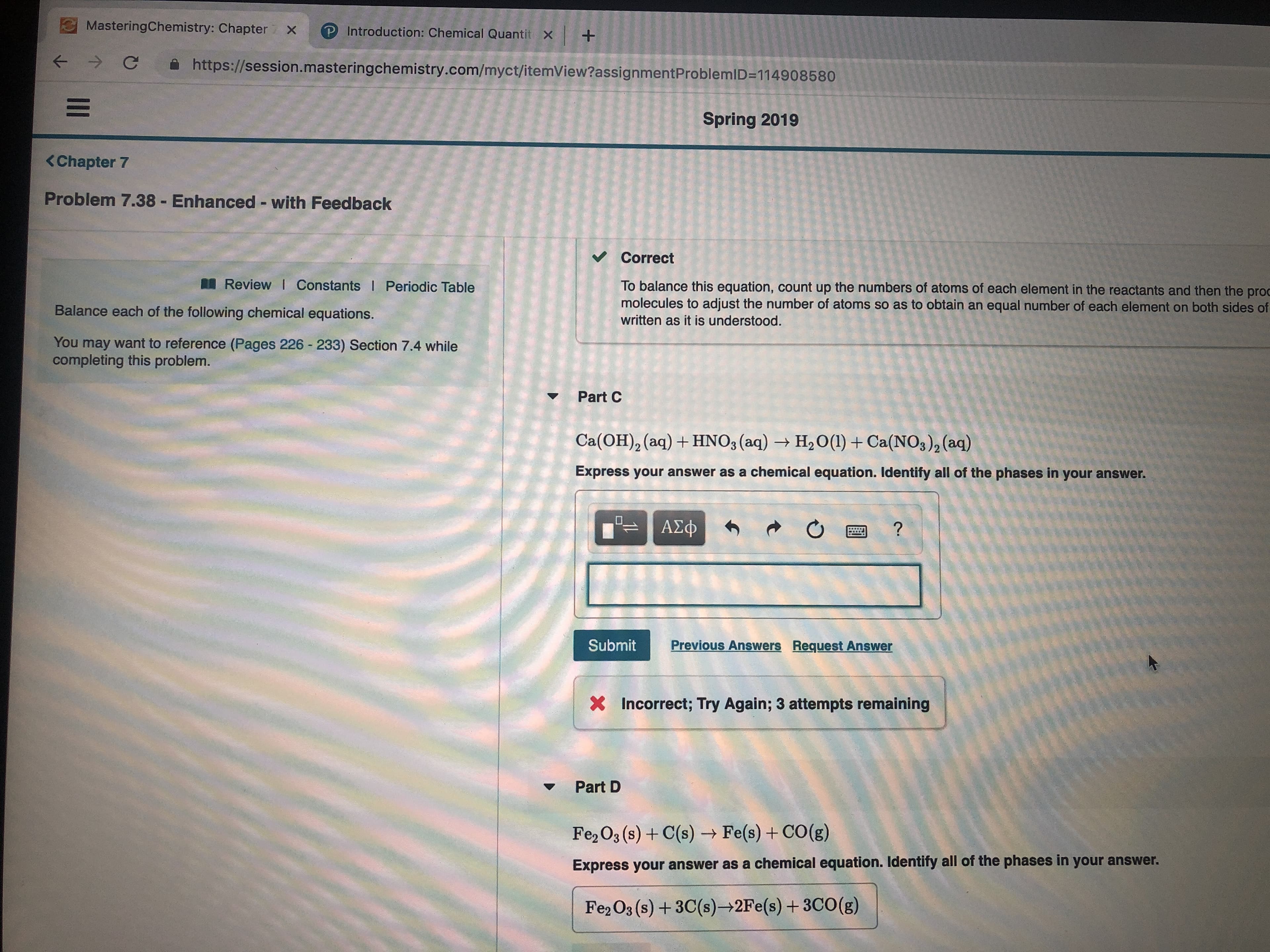
Problem 7.38 Enhanced - with Feedback
Correct
Review I Constants I Periodic Table
To balance this equation, count up the numbers of atoms of each element in the reactants and then the proc
molecules to adjust the number of atoms so as to obtain an equal number of each element on both sides of
Balance each of the following chemical equations.
written as it is understood.
You may want to reference (Pages 226 - 233) Section 7.4 while
completing this problem.
Part C
Ca(OH)2 (aq)HNO3 (aq)
H2O(1) Ca(NOs)2(aq)
Express your answer as a chemical equation. Identify all of the phases in your answer.
ΑΣφ
Previous Answers Request Answer
Submit
Incorrect; Try Again; 3 attempts remaining
X
Part D
Fe2 O3 (s)+C(s)- Fe(s)+CO(g)
Express your answer as a chemical equation. Identify all of the phases in your answer.
Fe2 O3 (s)+3C(s) 2Fe(s)+ 3CO(g)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

