Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter18: Electrochemistry
Section: Chapter Questions
Problem 9RQ: What characterizes an electrolytic cell? What is an ampere? When the current applied to an...
Related questions
Question
Transcribed Image Text:MasteringChemistry: CHE154 SX
session.masteringchemistry.com/myct/itemView?as
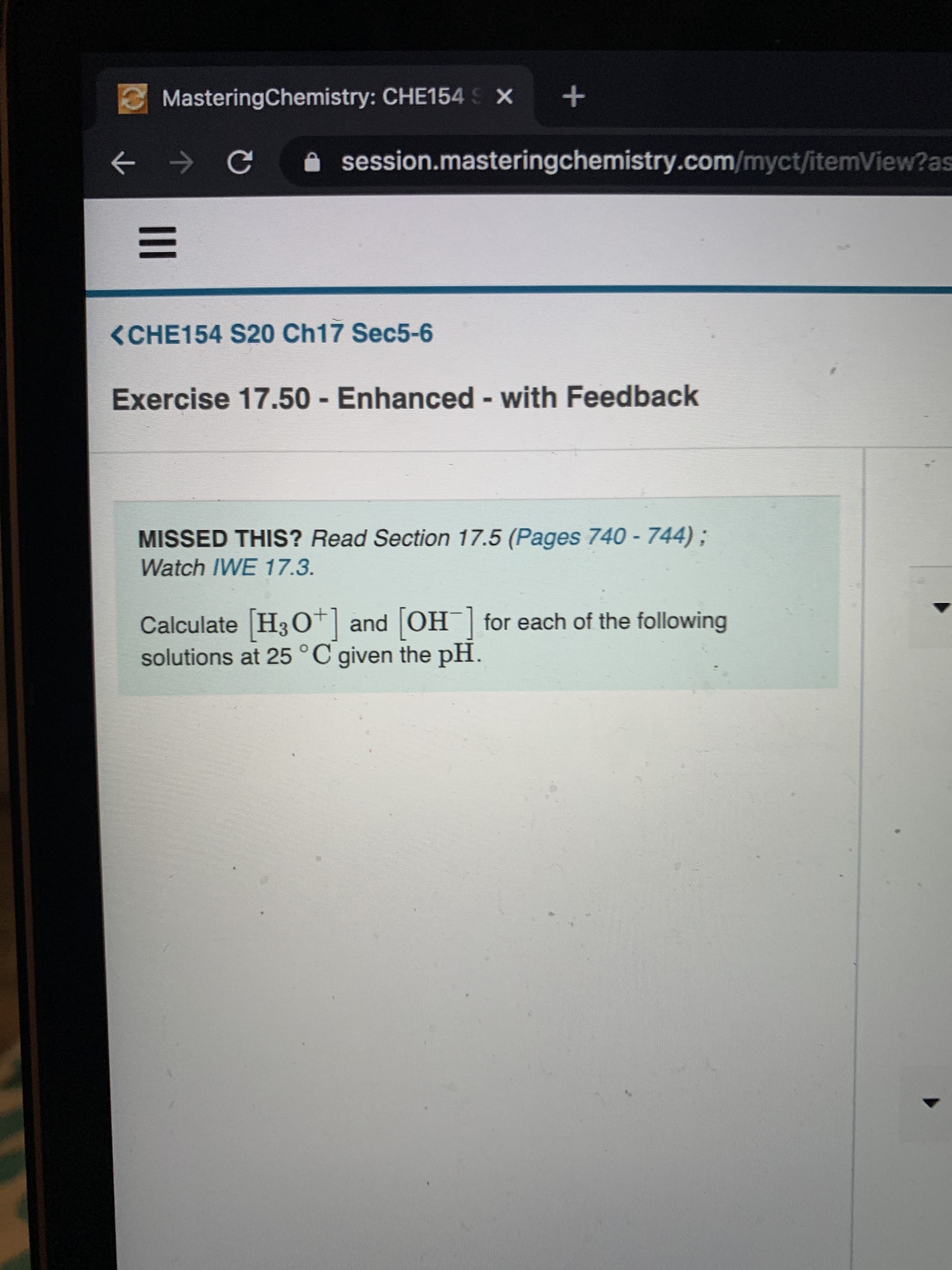
<CHE154 S20 Ch17 Sec5-6
Exercise 17.50 - Enhanced - with Feedback
MISSED THIS? Read Section 17.5 (Pages 740 - 744);
Watch IWE 17.3.
Calculate H3 O*| and OH for each of the following
solutions at 25°C given the pH.
![w?assignmentProblemlD3D142965856
CHE154-H Gen Chem II Bronikowski S20
IReview I
Part A
pH = 8.78
%3D
Express your answer using two significant figures. Enter your answers numerically separated by a comma.
ΑΣφ
[H3O*), [OH ] =
%3D
Submit
Request Answer
Part B](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F8ddcfb62-a75f-441b-9ba0-61b722369038%2F6dcc6be3-007b-4b09-9426-6d2bba7f0659%2Fjzdttzk.jpeg&w=3840&q=75)
Transcribed Image Text:w?assignmentProblemlD3D142965856
CHE154-H Gen Chem II Bronikowski S20
IReview I
Part A
pH = 8.78
%3D
Express your answer using two significant figures. Enter your answers numerically separated by a comma.
ΑΣφ
[H3O*), [OH ] =
%3D
Submit
Request Answer
Part B
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
