METALS T01/S03 The density of K is 862 cell is a whole number. kg/m3 and the cell volume is 1.51 x 10-22 mL. Determine the number of atoms in the unit cell. Note: the number of atoms in a unit Molar Mass (g/mol) K 39.098 Packing bcc body centered cubic ccp cubic close pack hcp hexagonal close pack Avogadro's No. (mol"1) 6.022x1023
METALS T01/S03 The density of K is 862 cell is a whole number. kg/m3 and the cell volume is 1.51 x 10-22 mL. Determine the number of atoms in the unit cell. Note: the number of atoms in a unit Molar Mass (g/mol) K 39.098 Packing bcc body centered cubic ccp cubic close pack hcp hexagonal close pack Avogadro's No. (mol"1) 6.022x1023
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter9: Liquids, Solids, And Materials
Section: Chapter Questions
Problem IIISP
Related questions
Question
Can you please answer this with serious detail and depth, as in every last little step explained as tho i were a 3rd grader because i am using this to cram study I havent paid attention all quarter!!
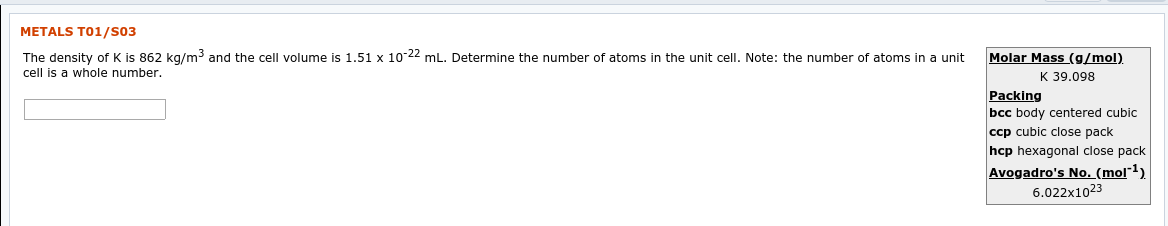
Transcribed Image Text:METALS T01/S03
The density of K is 862
cell is a whole number.
kg/m3 and the cell volume
is 1.51
x 10-22 mL. Determine the number of atoms in the unit cell. Note: the number of atoms in
a unit
Molar Mass (g/mol)
K 39.098
Packing
bcc body centered cubic
ccp cubic close pack
hcp hexagonal close pack
Avogadro's No. (mol"1)
6.022x1023
Expert Solution
Step 1
The number of atoms per unit cell, Z, is calculated as shown below where V is the volume of the unit cell, NA is the Avogadro’s number, d is the density of the unit cell, and M is the molar mass of the potassium.
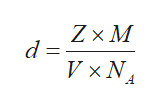
Step 2
The conversion for mL to m3 is shown below. Thus, 1.51×10-22 mL equal to 1.51×10-28 m3.
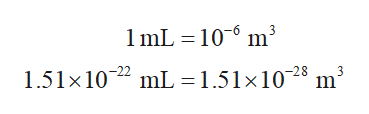
Step by step
Solved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
