Milliliters of isopropanol = mL Submit Part C How many liters of a 3.32 MK2SO4 solution are needed to provide 58.1 g of K2 SO4 (molar mass 174.01 g/mol)? Recall that M is equivalent to mol/L. Express your answer to th ree significant figures. View Available Hint(s) ? ΑΣΦ P Pearson Permissions Contact Us Copyright C2019 Pearson Education Inc. All rights reserved.| Terms of Use IPrivacy Policy F12 F11 II F8 F10 F9 F7 F6 F5 F4 F3 & A +II t
Milliliters of isopropanol = mL Submit Part C How many liters of a 3.32 MK2SO4 solution are needed to provide 58.1 g of K2 SO4 (molar mass 174.01 g/mol)? Recall that M is equivalent to mol/L. Express your answer to th ree significant figures. View Available Hint(s) ? ΑΣΦ P Pearson Permissions Contact Us Copyright C2019 Pearson Education Inc. All rights reserved.| Terms of Use IPrivacy Policy F12 F11 II F8 F10 F9 F7 F6 F5 F4 F3 & A +II t
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter13: The Chemistry Of Solutes And Solutions
Section: Chapter Questions
Problem 16QRT
Related questions
Question
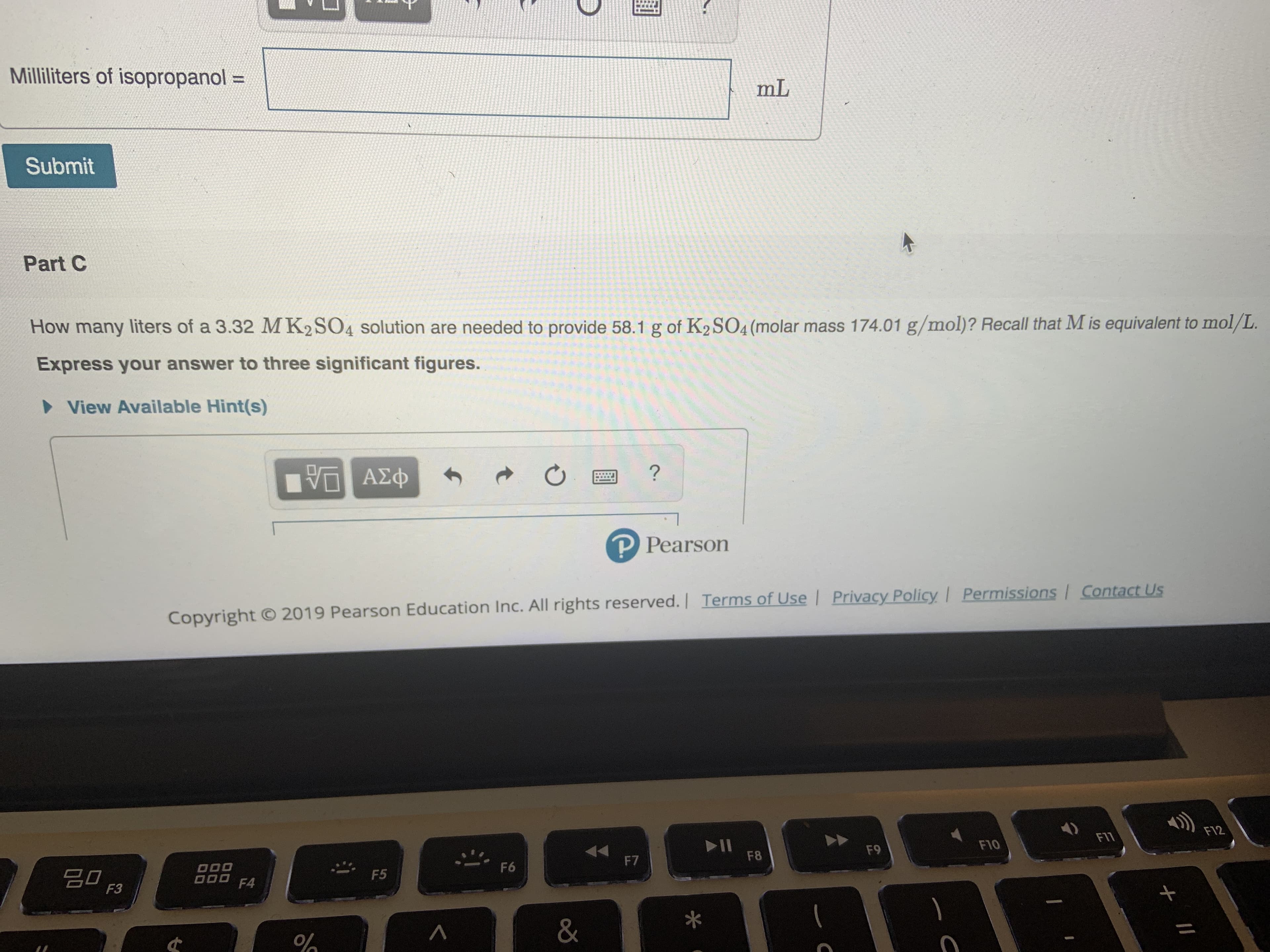
Transcribed Image Text:Milliliters of isopropanol =
mL
Submit
Part C
How many liters of a 3.32 MK2SO4 solution are needed to provide 58.1 g of K2 SO4 (molar mass 174.01 g/mol)? Recall that M is equivalent to mol/L.
Express your answer to th ree significant figures.
View Available Hint(s)
?
ΑΣΦ
P Pearson
Permissions Contact Us
Copyright C2019 Pearson Education Inc. All rights reserved.| Terms of Use IPrivacy Policy
F12
F11
II
F8
F10
F9
F7
F6
F5
F4
F3
&
A
+II
t
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 2 images

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

