MINDTAP Q Se (kJ) NO2 CO 358 226 NO CO2 1. Analyze Reaction Profile: This is group attempt 1 of 5 : Back ylla....docx x bp GE MINDTAP / NO CO2 Reaction Coordinate kJ What is the value of the activation energy for this reaction? Is this reaction exothermic or endothermic? What is the value of AE for this reaction? kJ Submit Ancwor 1. Analyze Reaction Profile: This is group attempt 1 of 5 ore: Back D%) A b Sylla....docx x ip
MINDTAP Q Se (kJ) NO2 CO 358 226 NO CO2 1. Analyze Reaction Profile: This is group attempt 1 of 5 : Back ylla....docx x bp GE MINDTAP / NO CO2 Reaction Coordinate kJ What is the value of the activation energy for this reaction? Is this reaction exothermic or endothermic? What is the value of AE for this reaction? kJ Submit Ancwor 1. Analyze Reaction Profile: This is group attempt 1 of 5 ore: Back D%) A b Sylla....docx x ip
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter13: Substitution
Section: Chapter Questions
Problem 43CTQ
Related questions
Question
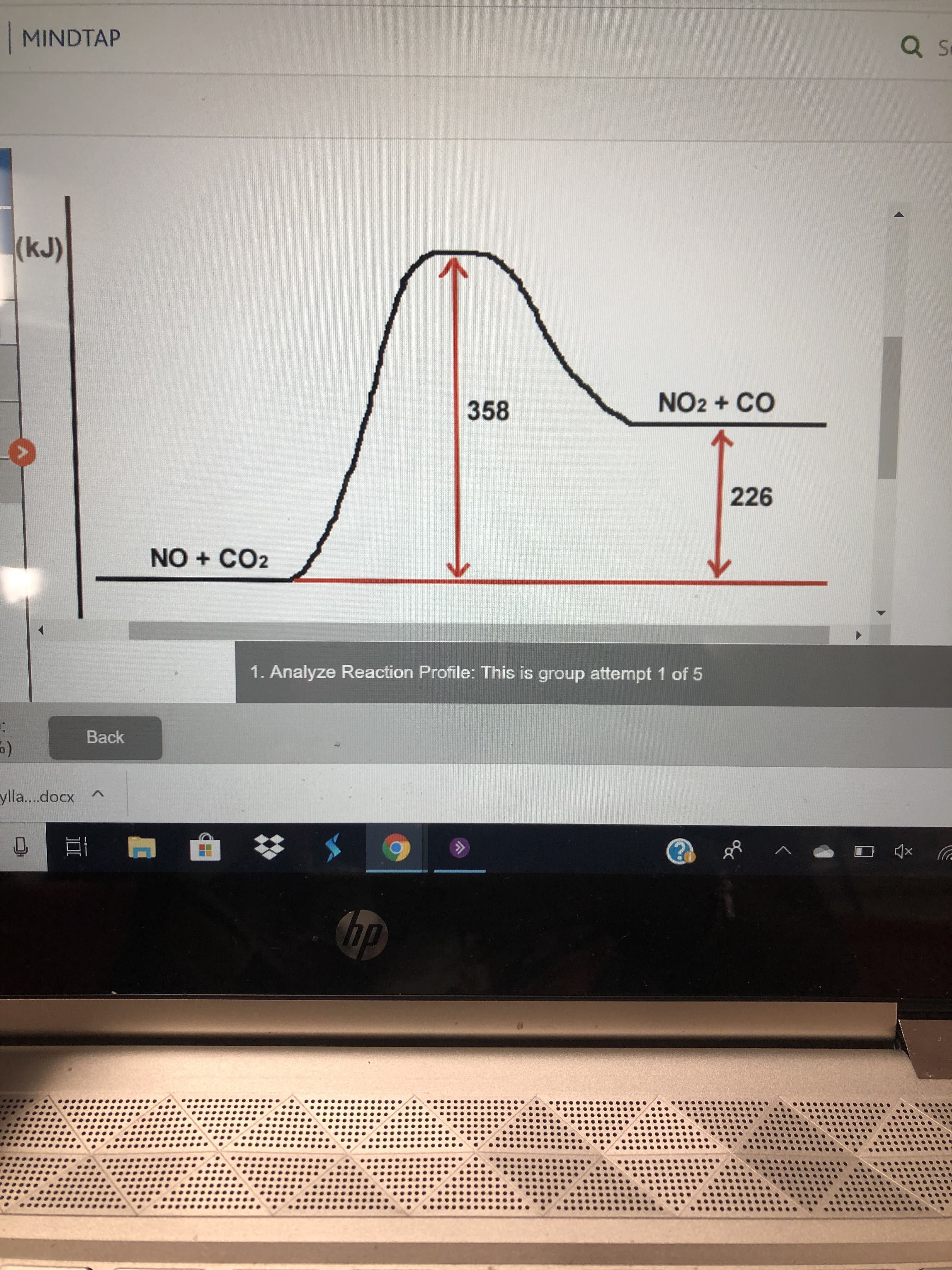
| What is the value of the activation energy for this reaction? _______kJ |
| Is this reaction exothermic or endothermic? _________ |
| What is the value of E for this reaction? _________kJ |
Transcribed Image Text:MINDTAP
Q Se
(kJ)
NO2 CO
358
226
NO CO2
1. Analyze Reaction Profile: This is group attempt 1 of 5
:
Back
ylla....docx
x
bp
Transcribed Image Text:GE MINDTAP
/
NO CO2
Reaction Coordinate
kJ
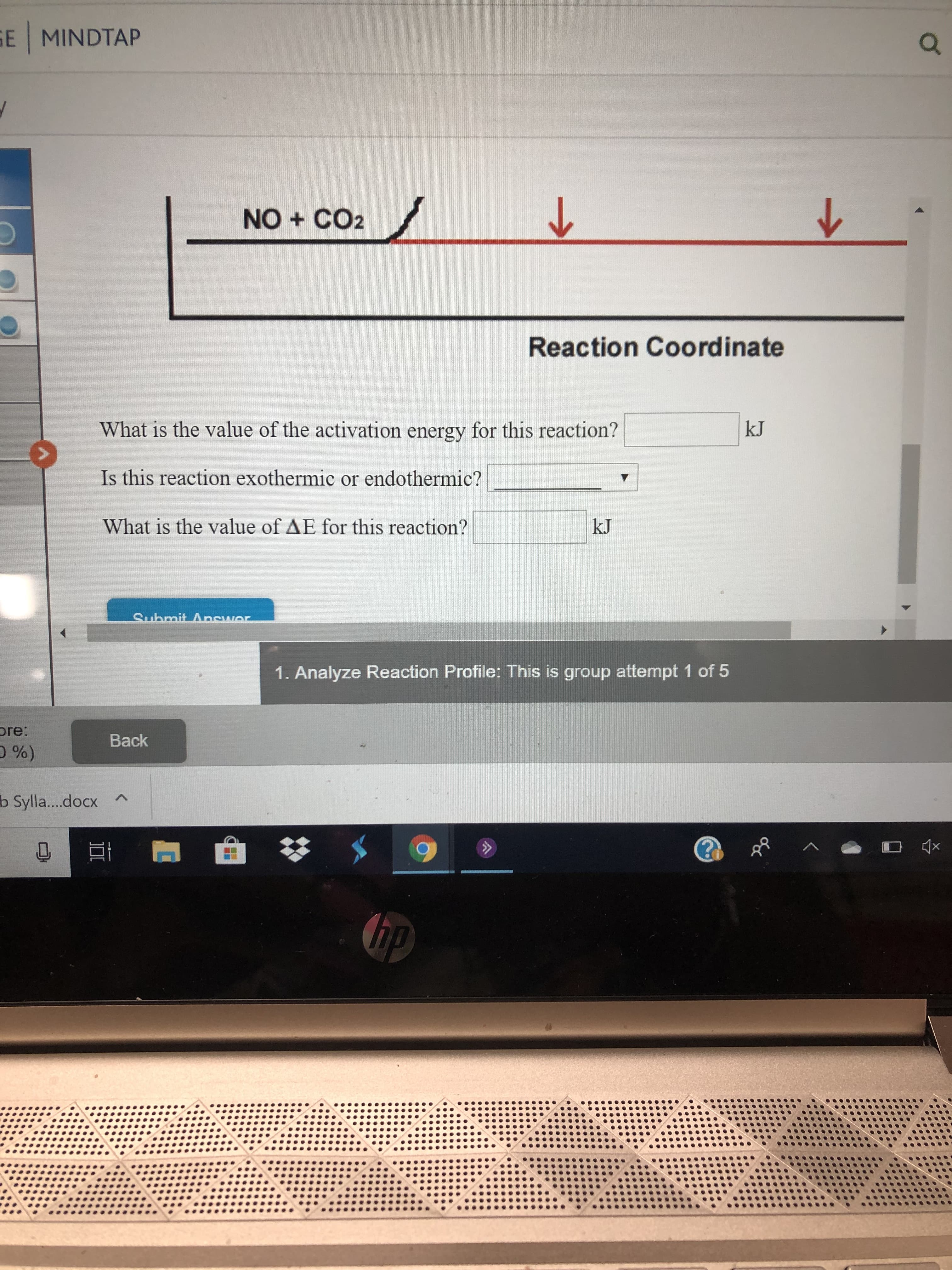
What is the value of the activation energy for this reaction?
Is this reaction exothermic or endothermic?
What is the value of AE for this reaction?
kJ
Submit Ancwor
1. Analyze Reaction Profile: This is group attempt 1 of 5
ore:
Back
D%)
A
b Sylla....docx
x
ip
Expert Solution
Step 1
(a)
Activation energy is defined as the minimum energy required by the reacting species in order to undergo chemical reaction. It is the energy difference between the transition state and reactants. Therefore, value of the activation energy for this reaction is 358 kJ.
Step 2
(b)
In the given energy profile graph, energy of product is higher than the energy of reactants. It implies that heat is absorbed during the reaction (ΔH is positive). Therefore, the given reaction is endothermic.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning