molecules by the action of the solvent. For example: HCI>H*(aq) + Cl'(aq) Hydrochloric acid (HCI) is an ionic compound that is broken down into two ions: Hydrogen (H*) and Chlorine (Cl). An ionic compound contains a cation with a positive charge (H*) and an anion with a negative charge (Cl). For a compound to go through ionization (be broken down into ions), it must be an ionic compound. Note that molecular compounds to not go through ionization. 1. What two classes of elements make up molecular compounds? 2. What classes of elements make up ionic compounds?
molecules by the action of the solvent. For example: HCI>H*(aq) + Cl'(aq) Hydrochloric acid (HCI) is an ionic compound that is broken down into two ions: Hydrogen (H*) and Chlorine (Cl). An ionic compound contains a cation with a positive charge (H*) and an anion with a negative charge (Cl). For a compound to go through ionization (be broken down into ions), it must be an ionic compound. Note that molecular compounds to not go through ionization. 1. What two classes of elements make up molecular compounds? 2. What classes of elements make up ionic compounds?
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter5: Thermochemistry
Section: Chapter Questions
Problem 5.101QE: In the 1880s, Frederick Trouton noted that the enthalpy of vaporization of 1 mol pure liquid is...
Related questions
Question
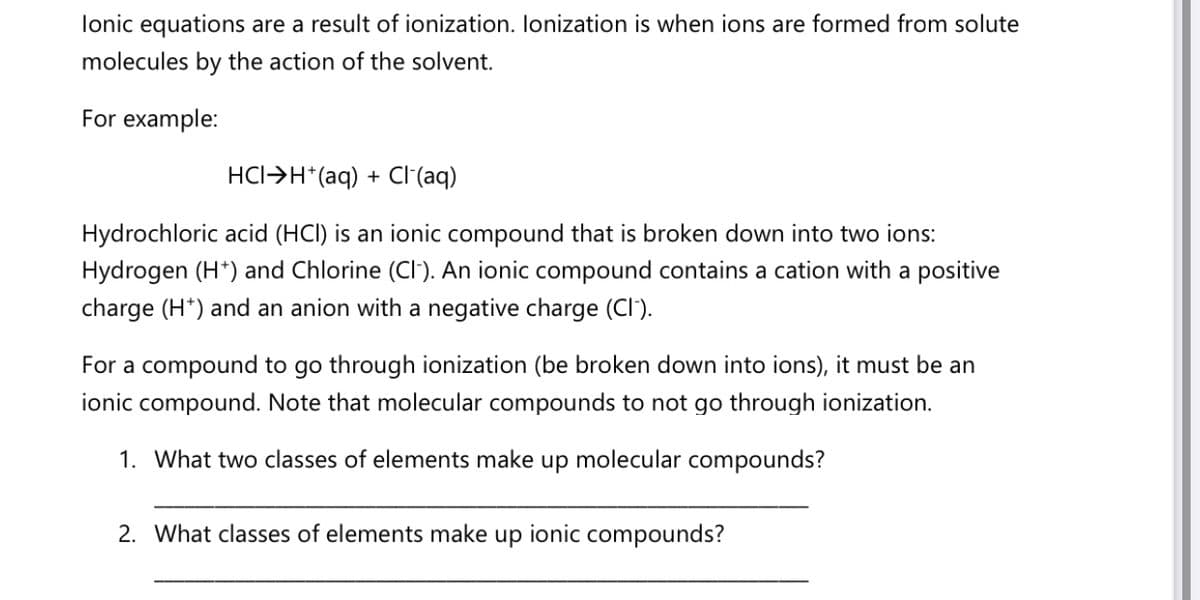
Transcribed Image Text:lonic equations are a result of ionization. Ionization is when ions are formed from solute
molecules by the action of the solvent.
For example:
HCI>H*(aq) + Cl(aq)
Hydrochloric acid (HCI) is an ionic compound that is broken down into two ions:
Hydrogen (H*) and Chlorine (CI'). An ionic compound contains a cation with a positive
charge (H*) and an anion with a negative charge (CI).
For a compound to go through ionization (be broken down into ions), it must be an
ionic compound. Note that molecular compounds to not go through ionization.
1. What two classes of elements make up molecular compounds?
2. What classes of elements make
dn
ionic compounds?
Expert Solution
Step 1
Classification of ionic and molecular compounds
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning