Molecules in a spherical container make wall collisions in a great circle plane of the sphere. All paths traveled between collisions are equal in length. (a) Relate the path length Al to the angle 0 and the sphere radius r. (b) Express the component of the molecular momentum transferred to the wall in a collision in terms of the molecular mass m, the speed v, and the angle 0. (c) Using parts (a) and (b), calculate the average force exerted on the wall by one molecule. (d) Derive the relation PV = PV = } Nmv² for a spheri- cal container. This is the same relation found for a rectangular box in Section 9.5.
Molecules in a spherical container make wall collisions in a great circle plane of the sphere. All paths traveled between collisions are equal in length. (a) Relate the path length Al to the angle 0 and the sphere radius r. (b) Express the component of the molecular momentum transferred to the wall in a collision in terms of the molecular mass m, the speed v, and the angle 0. (c) Using parts (a) and (b), calculate the average force exerted on the wall by one molecule. (d) Derive the relation PV = PV = } Nmv² for a spheri- cal container. This is the same relation found for a rectangular box in Section 9.5.
Related questions
Question
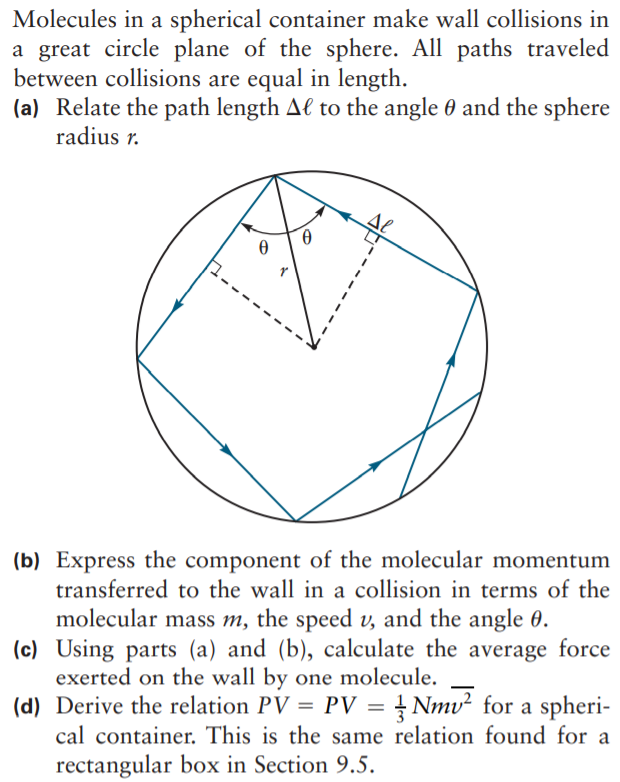
Transcribed Image Text:Molecules in a spherical container make wall collisions in
a great circle plane of the sphere. All paths traveled
between collisions are equal in length.
(a) Relate the path length Al to the angle 0 and the sphere
radius r.
(b) Express the component of the molecular momentum
transferred to the wall in a collision in terms of the
molecular mass m, the speed v, and the angle 0.
(c) Using parts (a) and (b), calculate the average force
exerted on the wall by one molecule.
(d) Derive the relation PV = PV = } Nmv² for a spheri-
cal container. This is the same relation found for a
rectangular box in Section 9.5.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images
