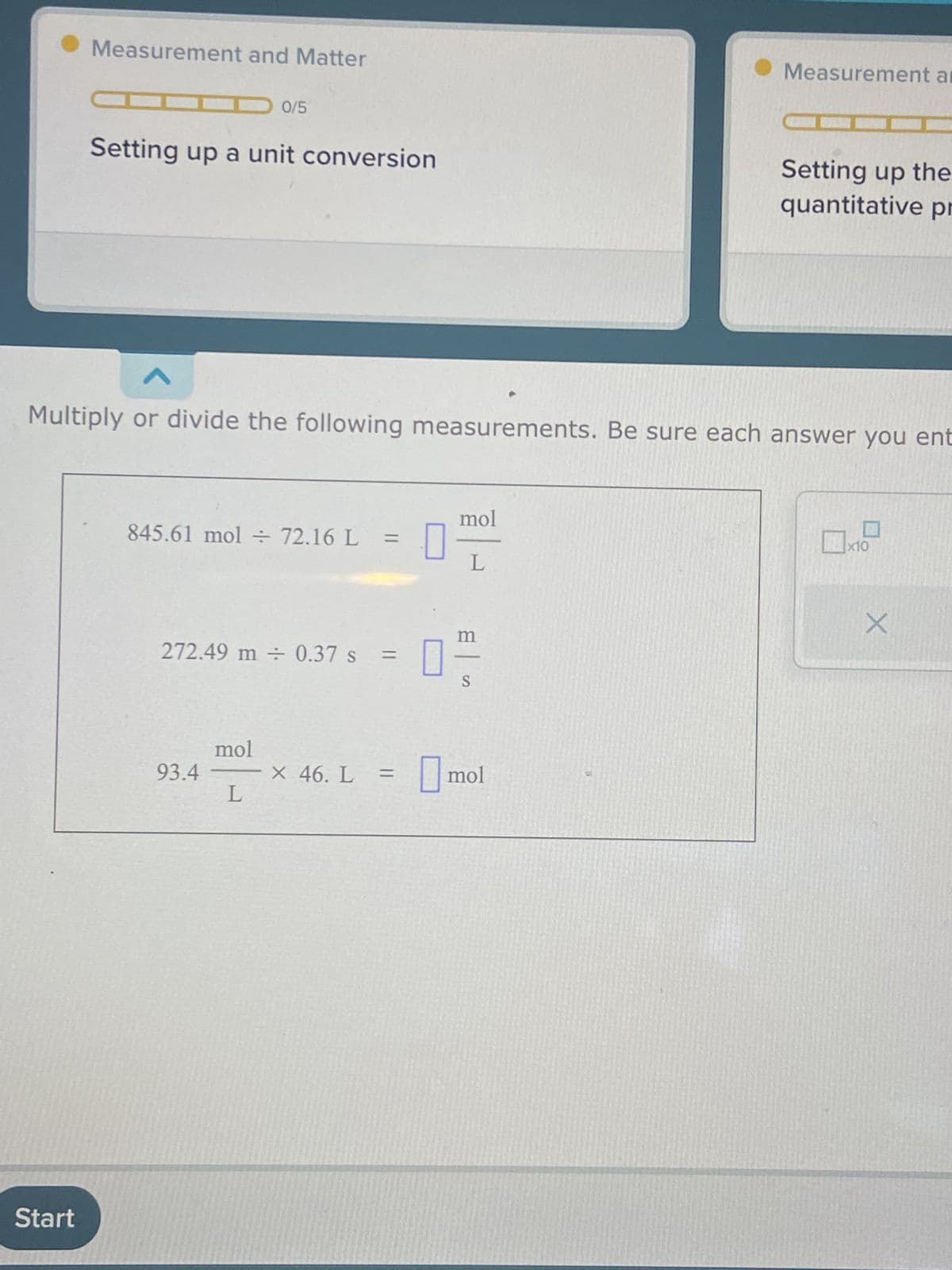
Multiply or divide the following measurements. Be sure each answer you ent 845.61 mol 72.16 L = 272.49 m 0.37 s : = 93.4 mol L x 46. L = mol m S mol x10 X
Multiply or divide the following measurements. Be sure each answer you ent 845.61 mol 72.16 L = 272.49 m 0.37 s : = 93.4 mol L x 46. L = mol m S mol x10 X
General, Organic, and Biological Chemistry
7th Edition
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:H. Stephen Stoker
Chapter2: Measurements In Chemistry
Section: Chapter Questions
Problem 2.102EP: Calculate the volume, in milliliters, for each of the following 66.0-g masses of substances. a. 66.0...
Related questions
Question
845.61 mol ÷ 72.16 L
Transcribed Image Text:Measurement and Matter
Start
Setting up a unit conversion
0/5
Multiply or divide the following measurements. Be sure each answer you ent
845.61 mol + 72.16 L = 0
272.49 m ÷ 0.37 s =
93.4
mol
L
× 46. L =
mol
L
m
0 고
S
mol
Measurement ar
3
Setting up the
quantitative pr
x10
X
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning