My morning cup of tea is steaming hot at 95°C. I take a 37 that has a temperature of -18°C. (Use specific heat for water for the tea.) ice cube from my freezer gg How much heat does it take to raise the temperature of the ice cube to melting? O How much heat does it take to melt the ice cube? How much tea do I need to equilibrate at my ideal tea drinking temperature of O 67.4°C? Ta Specific Heat Char Heat of cal/g °C Vaporization Heat of Fusion or Melting Boiling cal/g cal/g kcal/kg °C Point Point or or or (°C) (°C) J/kg kcal/kg Btu/lb °F J/kg J/kg °C kcal/kg Alcohol, ethyl Aluminum -117 1.04 105 3.21 105 8.54X 10 78.5 0.58 204 2400 24.9 660 2057 0.22 920 76.8 Brass 840 0.092 390 Сopper 1083 2330 0.092 390 49.0 2.05 x 105 Glass 0.21 880 Ice 0 0.51 2100 80 3.35 x 105 Iron (steel) 1540 3000 0.115 481 7.89 3.30 X 10 2.45 10 1.18 x 10 1.09 105 Lead 327 1620 0.031 130 5.86 Mercury -38.9 357 0.033 140 2.82 65.0 2.72 X 105 Silver 961 1950 0.056 230 26.0 Steam 0.48 2000 Water 100 (liquid) 1.00 4190 0 3.35 105 80 540 2.26 x 106 Zinc 907 0.092 419 390 23.0 9.63 X 104
My morning cup of tea is steaming hot at 95°C. I take a 37 that has a temperature of -18°C. (Use specific heat for water for the tea.) ice cube from my freezer gg How much heat does it take to raise the temperature of the ice cube to melting? O How much heat does it take to melt the ice cube? How much tea do I need to equilibrate at my ideal tea drinking temperature of O 67.4°C? Ta Specific Heat Char Heat of cal/g °C Vaporization Heat of Fusion or Melting Boiling cal/g cal/g kcal/kg °C Point Point or or or (°C) (°C) J/kg kcal/kg Btu/lb °F J/kg J/kg °C kcal/kg Alcohol, ethyl Aluminum -117 1.04 105 3.21 105 8.54X 10 78.5 0.58 204 2400 24.9 660 2057 0.22 920 76.8 Brass 840 0.092 390 Сopper 1083 2330 0.092 390 49.0 2.05 x 105 Glass 0.21 880 Ice 0 0.51 2100 80 3.35 x 105 Iron (steel) 1540 3000 0.115 481 7.89 3.30 X 10 2.45 10 1.18 x 10 1.09 105 Lead 327 1620 0.031 130 5.86 Mercury -38.9 357 0.033 140 2.82 65.0 2.72 X 105 Silver 961 1950 0.056 230 26.0 Steam 0.48 2000 Water 100 (liquid) 1.00 4190 0 3.35 105 80 540 2.26 x 106 Zinc 907 0.092 419 390 23.0 9.63 X 104
Physics for Scientists and Engineers: Foundations and Connections
1st Edition
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Katz, Debora M.
Chapter21: Heat And The First Law Of Thermodynamics
Section: Chapter Questions
Problem 24PQ
Related questions
Question
Had this answered before, but I'm still confused... here is the table from the book.

Transcribed Image Text:My morning cup of tea is steaming hot at 95°C. I take a 37
that has a temperature of -18°C. (Use specific heat for water for the tea.)
ice cube from my freezer
gg
How much heat does it take to raise the temperature of the ice cube to melting?
O
How much heat does it take to melt the ice cube?
How much tea do I need to equilibrate at my ideal tea drinking temperature of
O
67.4°C?
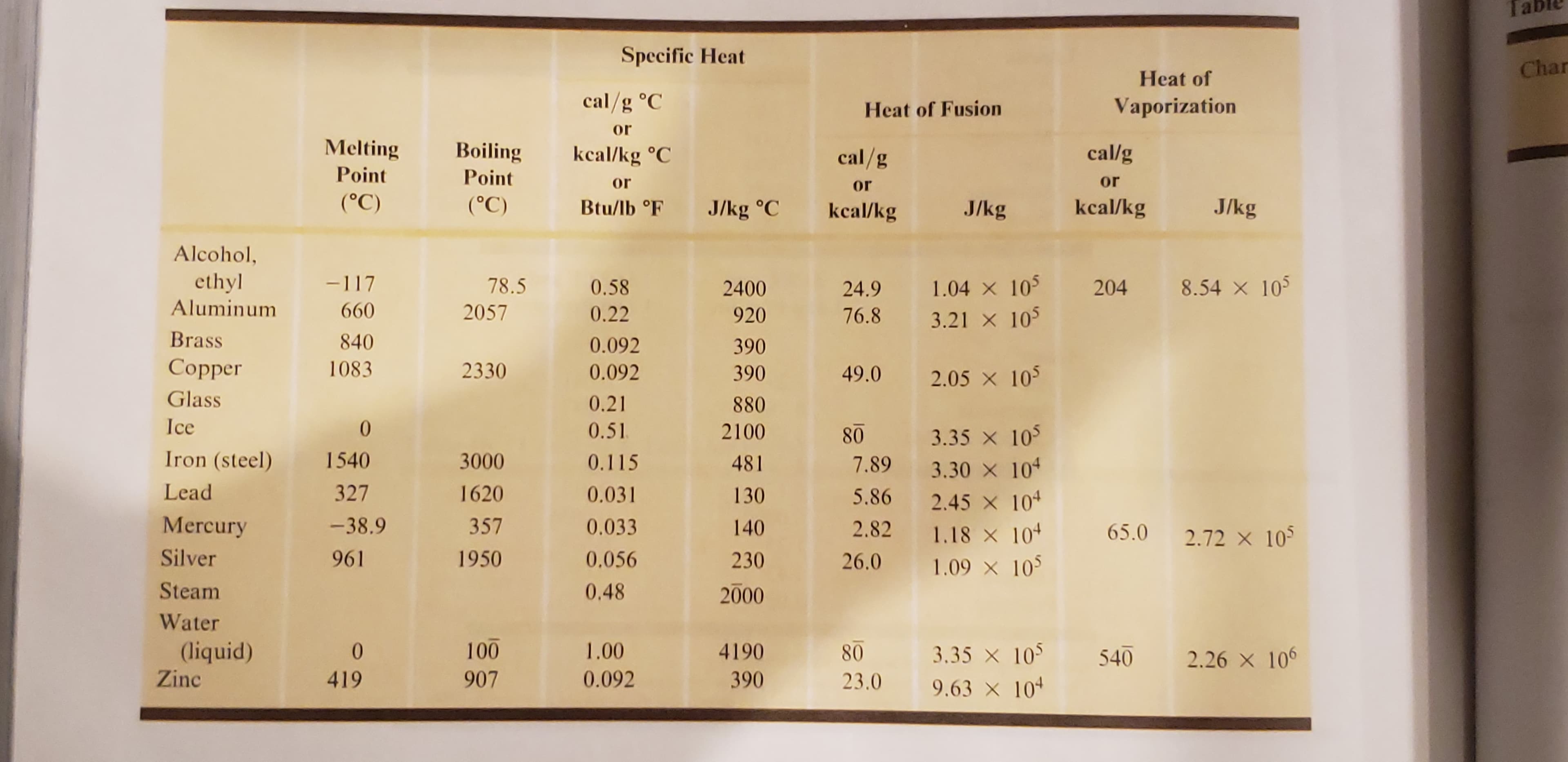
Transcribed Image Text:Ta
Specific Heat
Char
Heat of
cal/g °C
Vaporization
Heat of Fusion
or
Melting
Boiling
cal/g
cal/g
kcal/kg °C
Point
Point
or
or
or
(°C)
(°C)
J/kg
kcal/kg
Btu/lb °F
J/kg
J/kg °C
kcal/kg
Alcohol,
ethyl
Aluminum
-117
1.04 105
3.21 105
8.54X 10
78.5
0.58
204
2400
24.9
660
2057
0.22
920
76.8
Brass
840
0.092
390
Сopper
1083
2330
0.092
390
49.0
2.05 x 105
Glass
0.21
880
Ice
0
0.51
2100
80
3.35 x 105
Iron (steel)
1540
3000
0.115
481
7.89
3.30 X 10
2.45 10
1.18 x 10
1.09 105
Lead
327
1620
0.031
130
5.86
Mercury
-38.9
357
0.033
140
2.82
65.0
2.72 X 105
Silver
961
1950
0.056
230
26.0
Steam
0.48
2000
Water
100
(liquid)
1.00
4190
0
3.35 105
80
540
2.26 x 106
Zinc
907
0.092
419
390
23.0
9.63 X 104
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning


College Physics
Physics
ISBN:
9781305952300
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning


College Physics
Physics
ISBN:
9781305952300
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College