n = 5 Ionized atom n = 4 (continuous energy levels) E = 0 - 0.85 n= 3 Excited -1.5 states Paschen series -3.4 Tn=2 FIGURE 27-29 Energy-level diagram for the hydrogen atom, showing the transitions for the spectral lines of the Lyman, Balmer, and Paschen series (Fig. 27-25). Each vertical arrow represents an atomic transition that gives rise to the photons of one spectral line (a single wavelength or frequency). Balmer -5 series -10 n = 1 Ground state -13.6 Lyman series -15 Energy (eV)
n = 5 Ionized atom n = 4 (continuous energy levels) E = 0 - 0.85 n= 3 Excited -1.5 states Paschen series -3.4 Tn=2 FIGURE 27-29 Energy-level diagram for the hydrogen atom, showing the transitions for the spectral lines of the Lyman, Balmer, and Paschen series (Fig. 27-25). Each vertical arrow represents an atomic transition that gives rise to the photons of one spectral line (a single wavelength or frequency). Balmer -5 series -10 n = 1 Ground state -13.6 Lyman series -15 Energy (eV)
Modern Physics
3rd Edition
ISBN:9781111794378
Author:Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Publisher:Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Chapter4: The Particle Nature Of Matter
Section: Chapter Questions
Problem 26P
Related questions
Question
Construct the energy-level diagram for the He+ ion
(like Fig. 27–29).
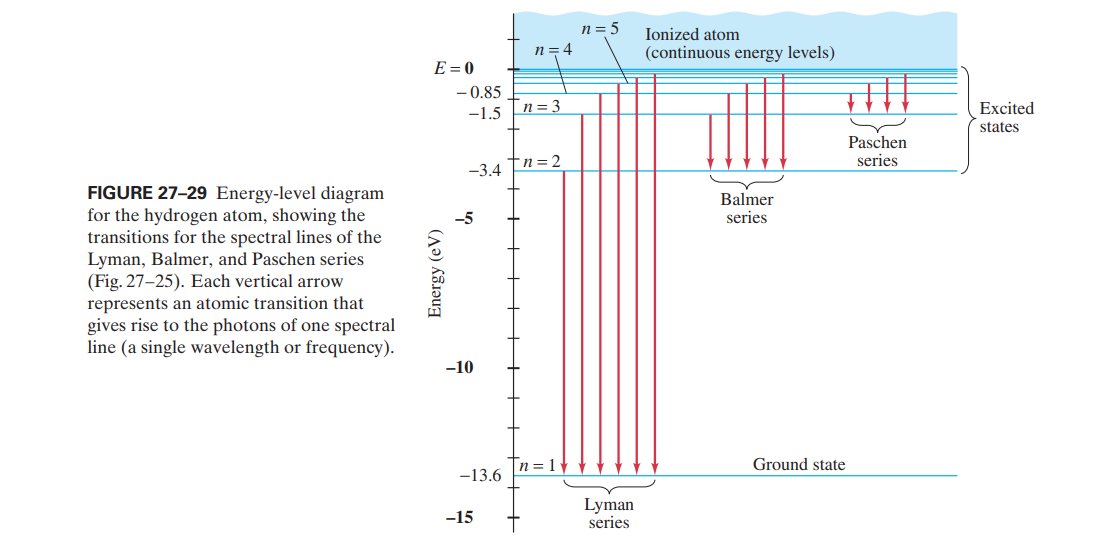
Transcribed Image Text:n = 5
Ionized atom
n = 4
(continuous energy levels)
E = 0
- 0.85
n= 3
Excited
-1.5
states
Paschen
series
-3.4 Tn=2
FIGURE 27-29 Energy-level diagram
for the hydrogen atom, showing the
transitions for the spectral lines of the
Lyman, Balmer, and Paschen series
(Fig. 27-25). Each vertical arrow
represents an atomic transition that
gives rise to the photons of one spectral
line (a single wavelength or frequency).
Balmer
-5
series
-10
n = 1
Ground state
-13.6
Lyman
series
-15
Energy (eV)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

Modern Physics
Physics
ISBN:
9781111794378
Author:
Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Publisher:
Cengage Learning


Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Modern Physics
Physics
ISBN:
9781111794378
Author:
Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Publisher:
Cengage Learning


Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

University Physics Volume 3
Physics
ISBN:
9781938168185
Author:
William Moebs, Jeff Sanny
Publisher:
OpenStax

College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College