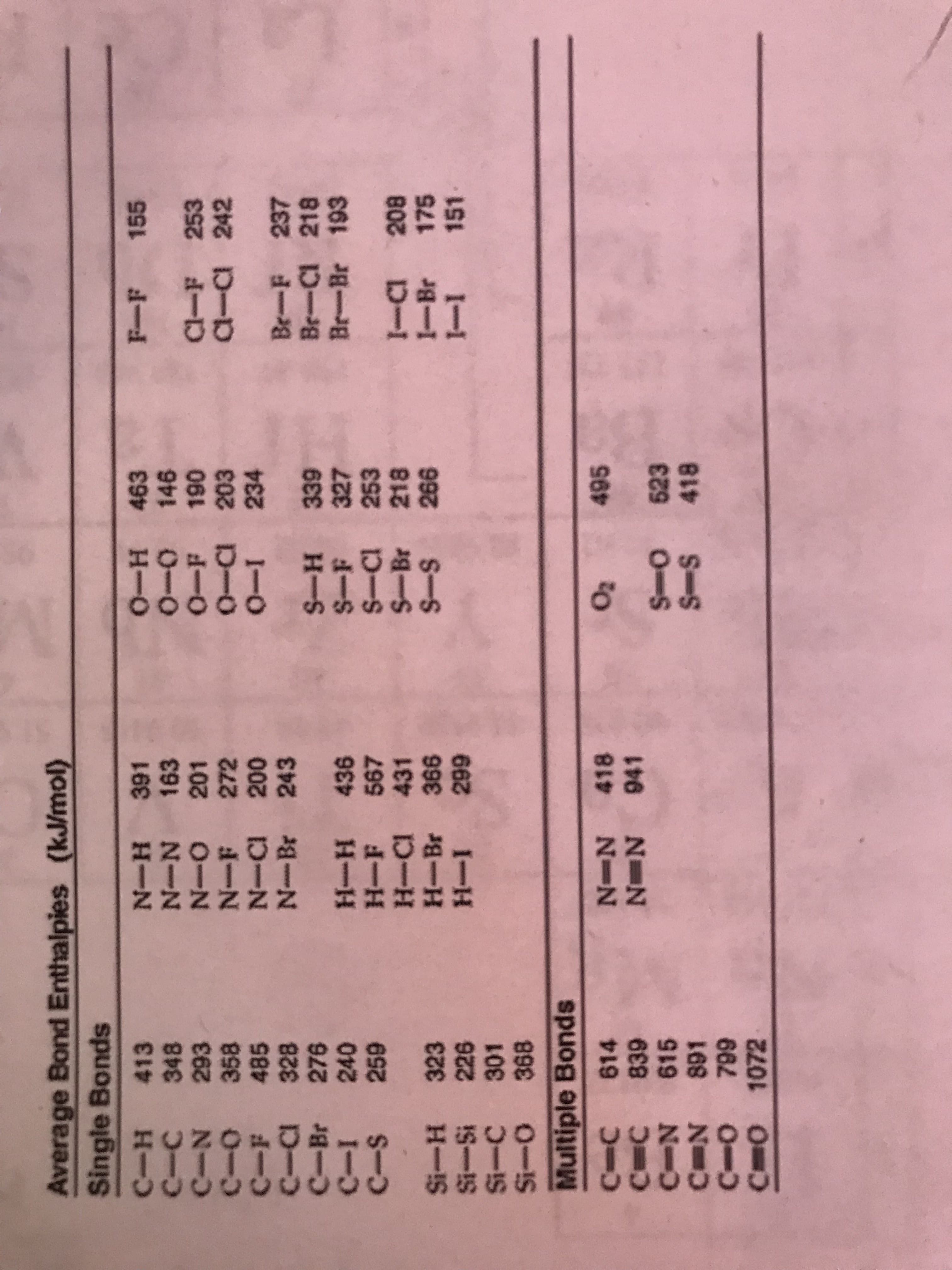
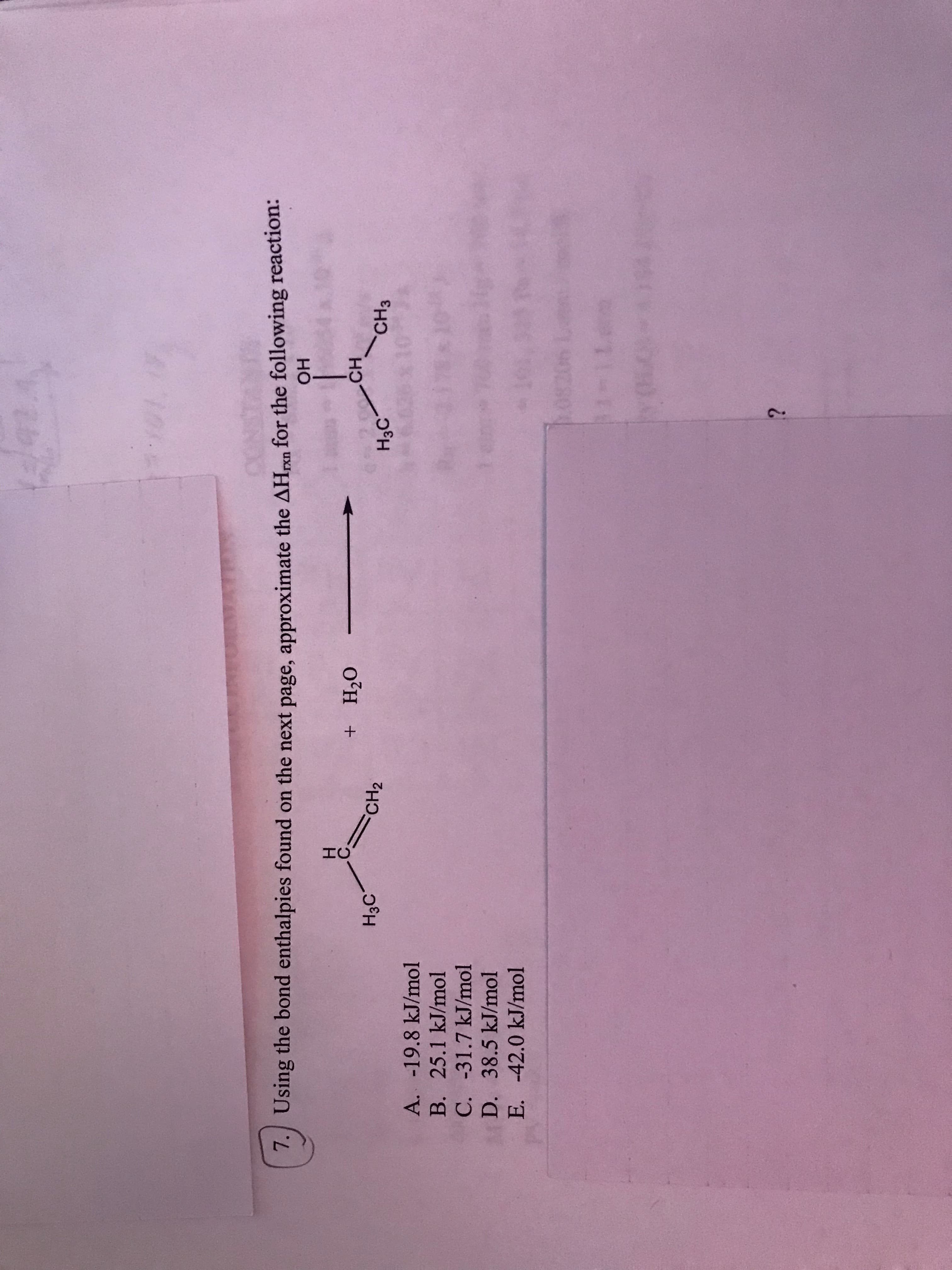
N Average Bond Enthalpies (kJ/mol) Single Bonds C-H 413 C-C 348 H-O 463 N-H 391 N-N 163 N-O 201 N-F 272 N-CI 200 N-Br 243 155 O-o 146 060 F 190 N 293 N-O Cl-F 253 a-a 242 C-o 358 485 C-a 328 C-Br 276 240 1- 0-a 203 0- 234 Br-F 237 Br-Ci 218 Br-Br 193 H-S S-F 327 S-CI 253 S-Br 218 259 9 334 H-F 567 H-CI 431 H-Br 366 -a 208 1-Br 175 151 1-1 Si-H 323 Si-Si 226 Si-C 301 Si-O 368 S-S 926 I-H Multiple Bonds 495 N-N 418 N N 941 IO 614 C C 839 C-N 615 C N 891 C-o 799 C O 1072 S-o 623 S-S 418 101 Using the bond enthalpies found on the next page, approximate the AHpxn for the following reaction: но Но CH3 H3C CH2 о'н H3C A. -19.8 kJ/mol B. 25.1 kJ/mol C. -31.7 kJ/mol *84 D. 38.5 kJ/mol E. -42.0 kJ/mol 1-0
N Average Bond Enthalpies (kJ/mol) Single Bonds C-H 413 C-C 348 H-O 463 N-H 391 N-N 163 N-O 201 N-F 272 N-CI 200 N-Br 243 155 O-o 146 060 F 190 N 293 N-O Cl-F 253 a-a 242 C-o 358 485 C-a 328 C-Br 276 240 1- 0-a 203 0- 234 Br-F 237 Br-Ci 218 Br-Br 193 H-S S-F 327 S-CI 253 S-Br 218 259 9 334 H-F 567 H-CI 431 H-Br 366 -a 208 1-Br 175 151 1-1 Si-H 323 Si-Si 226 Si-C 301 Si-O 368 S-S 926 I-H Multiple Bonds 495 N-N 418 N N 941 IO 614 C C 839 C-N 615 C N 891 C-o 799 C O 1072 S-o 623 S-S 418 101 Using the bond enthalpies found on the next page, approximate the AHpxn for the following reaction: но Но CH3 H3C CH2 о'н H3C A. -19.8 kJ/mol B. 25.1 kJ/mol C. -31.7 kJ/mol *84 D. 38.5 kJ/mol E. -42.0 kJ/mol 1-0
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter9: Energy And Chemistry
Section: Chapter Questions
Problem 9.77PAE: 9.77 When a reaction is exothermic, is the sum of bond energies of products or of reactants greater?
Related questions
Question
Transcribed Image Text:N
Average Bond Enthalpies (kJ/mol)
Single Bonds
C-H 413
C-C 348
H-O
463
N-H 391
N-N 163
N-O 201
N-F 272
N-CI 200
N-Br 243
155
O-o 146
060
F 190
N 293
N-O
Cl-F 253
a-a 242
C-o 358
485
C-a 328
C-Br 276
240
1-
0-a 203
0- 234
Br-F 237
Br-Ci 218
Br-Br 193
H-S
S-F 327
S-CI 253
S-Br 218
259
9 334
H-F 567
H-CI 431
H-Br 366
-a 208
1-Br 175
151
1-1
Si-H 323
Si-Si 226
Si-C 301
Si-O 368
S-S
926
I-H
Multiple Bonds
495
N-N 418
N N 941
IO
614
C C 839
C-N 615
C N 891
C-o 799
C O 1072
S-o 623
S-S 418
Transcribed Image Text:101
Using the bond enthalpies found on the next page, approximate the AHpxn for the following reaction:
но
Но
CH3
H3C
CH2
о'н
H3C
A. -19.8 kJ/mol
B. 25.1 kJ/mol
C. -31.7 kJ/mol
*84
D. 38.5 kJ/mol
E. -42.0 kJ/mol
1-0
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning