When a hydrogen atom absorbs a photon of
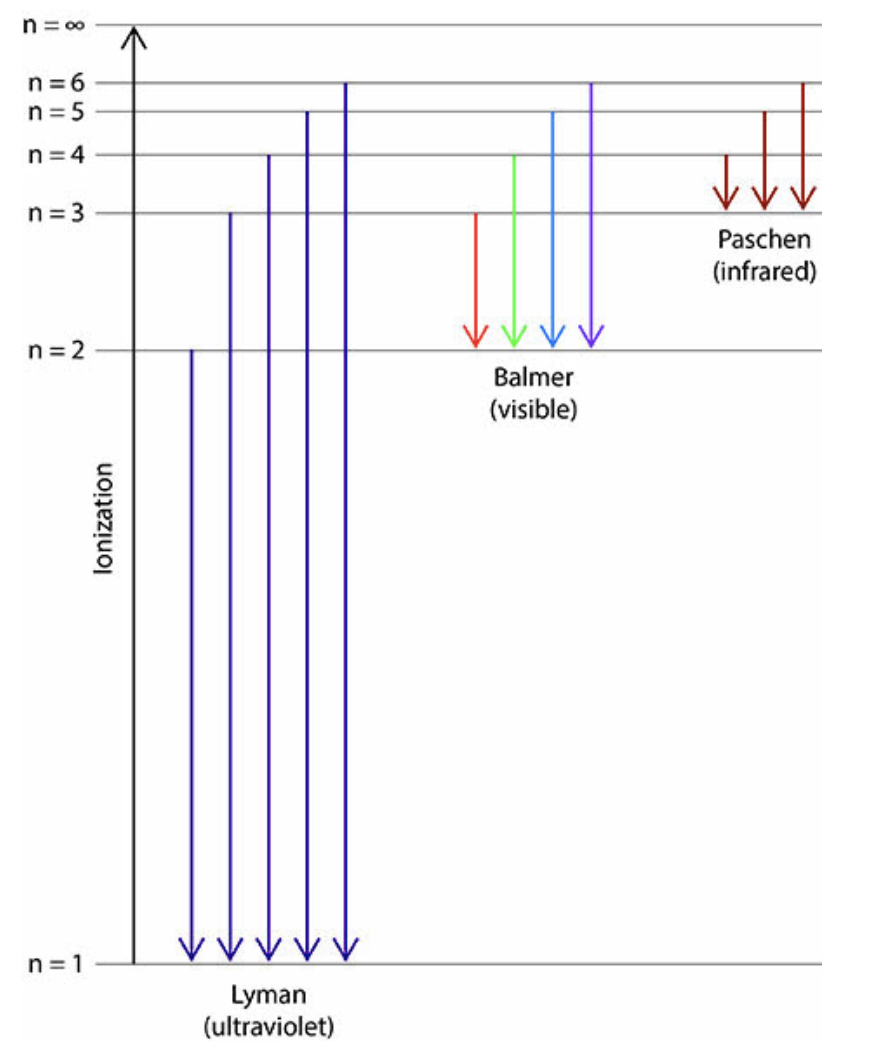
*refer to attached image before proceeding*
To ionize a hydrogen atom (forming a hydrogen ion), requires that the energy absorbed is sufficient to send the energized electron an infinite distance away from the nucleus (as shown by the ionization line in the image above). In other words, nfinal is equal to infinity.
Calculate the energy required to ionize a ground state hydrogen atom. Report your answer in kilojoules.
delta E= ____kJ
What is the longest wavelength of electromagnetic radiation capable of ionizing a ground state hydrogen atom? Report your answer in nanometers.
? = ______nm
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. Report your answer in kilojoules.
delta E= ____ kJ
What is the longest wavelength of electromagnetic radiation capable of ionizing this hydrogen atom in an excited state? Report your answer in nanometers.
? = ______nm
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 4 images






