name: 4. Electron transfer in the mitochondrial respiratory chain may be represented by the net reaction equation H20 + NAD* NADH + H* + 02 (a) Calculate AE'° for the net reaction of mitochondrial electron transfer. Use E'° values from Table 13-7. sinag Yn t noroest lierodT ITA did XNY) oalote dalipe A TAlo ano b Mt lln s (b) Calculate AG° for this reaction. -&.314 fodg ohnu iM (xMY) (c) How many ATP molecules can theoretically be generated by this reaction if the free energy of ATP synthesis under cellular conditions is 52 kJ/mol? ono l ban 9IA TTA TABLE 13-7b Standard Reduction Potentials of Some Biologically Important Half- Reactions Half-reaction E (V) Pyruvate+ 2H + 2e + lactate -0.185 Acetaldehyde + 2H* + 2e → ethanol FAD + 2H+ + 20 FADH2 -0.197 -0.219 Glutathione + 2H + 2e S+ 2H* + 2e+ H;S 2 reduced glutathione -0.23 -0.243 Lipoic acid + 2H* + 2e → dihydrolipoic acid -0.29 NAD* + H* + 2e NADH -0.320 NADP + H* + 2e + NADPH -0.324 Acetoacetate + 2H* + 2e > B-hydroxybutyrate a-Ketoglutarate + CO2 + 2H* + 2er + isocitrate 2H + 2e > H2 (at pH 7) Ferredoxin (Fe3*) + e> ferredoxin (Fe2") -0.346 4. -0.38 -0.414 -0.432 Source: Data mostly from R. A. Loach, in Handbook of Biochemistry and Molecular Biology, 3rd edn (G. D. Fasman, ed.), Physical and Chemical Data, Vol. 1, p. 122, CRC Press, 1976. SThis is the value for free FAD; FAD bound to a specific flavoprotein (e.g. succinate dehydrogenase) has a ditferent E" that depends on its protein environment.
name: 4. Electron transfer in the mitochondrial respiratory chain may be represented by the net reaction equation H20 + NAD* NADH + H* + 02 (a) Calculate AE'° for the net reaction of mitochondrial electron transfer. Use E'° values from Table 13-7. sinag Yn t noroest lierodT ITA did XNY) oalote dalipe A TAlo ano b Mt lln s (b) Calculate AG° for this reaction. -&.314 fodg ohnu iM (xMY) (c) How many ATP molecules can theoretically be generated by this reaction if the free energy of ATP synthesis under cellular conditions is 52 kJ/mol? ono l ban 9IA TTA TABLE 13-7b Standard Reduction Potentials of Some Biologically Important Half- Reactions Half-reaction E (V) Pyruvate+ 2H + 2e + lactate -0.185 Acetaldehyde + 2H* + 2e → ethanol FAD + 2H+ + 20 FADH2 -0.197 -0.219 Glutathione + 2H + 2e S+ 2H* + 2e+ H;S 2 reduced glutathione -0.23 -0.243 Lipoic acid + 2H* + 2e → dihydrolipoic acid -0.29 NAD* + H* + 2e NADH -0.320 NADP + H* + 2e + NADPH -0.324 Acetoacetate + 2H* + 2e > B-hydroxybutyrate a-Ketoglutarate + CO2 + 2H* + 2er + isocitrate 2H + 2e > H2 (at pH 7) Ferredoxin (Fe3*) + e> ferredoxin (Fe2") -0.346 4. -0.38 -0.414 -0.432 Source: Data mostly from R. A. Loach, in Handbook of Biochemistry and Molecular Biology, 3rd edn (G. D. Fasman, ed.), Physical and Chemical Data, Vol. 1, p. 122, CRC Press, 1976. SThis is the value for free FAD; FAD bound to a specific flavoprotein (e.g. succinate dehydrogenase) has a ditferent E" that depends on its protein environment.
Chapter29: The Organic Chemistry Of Metabolic Pathways
Section29.SE: Something Extra
Problem 50AP
Related questions
Question

Transcribed Image Text:name:
4. Electron transfer in the mitochondrial respiratory chain may be represented by the net
reaction equation
H20 + NAD*
NADH + H* + 02
(a) Calculate AE'° for the net reaction of mitochondrial electron transfer. Use E'° values from Table
13-7.
sinag Yn
t noroest lierodT
ITA did
XNY) oalote
dalipe
A TAlo ano
b
Mt lln s
(b) Calculate AG° for this reaction.
-&.314
fodg ohnu iM
(xMY)
(c) How many ATP molecules can theoretically be generated by this reaction if the free energy of
ATP synthesis under cellular conditions is 52 kJ/mol?
ono l ban 9IA TTA
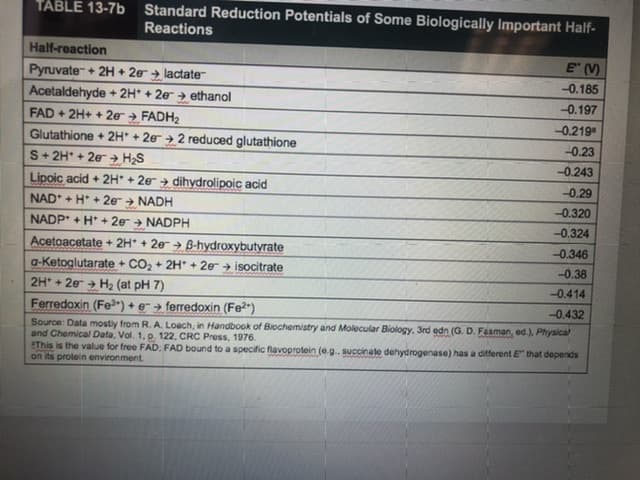
Transcribed Image Text:TABLE 13-7b
Standard Reduction Potentials of Some Biologically Important Half-
Reactions
Half-reaction
E (V)
Pyruvate+ 2H + 2e + lactate
-0.185
Acetaldehyde + 2H* + 2e → ethanol
FAD + 2H+ + 20 FADH2
-0.197
-0.219
Glutathione + 2H + 2e
S+ 2H* + 2e+ H;S
2 reduced glutathione
-0.23
-0.243
Lipoic acid + 2H* + 2e → dihydrolipoic acid
-0.29
NAD* + H* + 2e
NADH
-0.320
NADP + H* + 2e + NADPH
-0.324
Acetoacetate + 2H* + 2e > B-hydroxybutyrate
a-Ketoglutarate + CO2 + 2H* + 2er + isocitrate
2H + 2e > H2 (at pH 7)
Ferredoxin (Fe3*) + e> ferredoxin (Fe2")
-0.346
4.
-0.38
-0.414
-0.432
Source: Data mostly from R. A. Loach, in Handbook of Biochemistry and Molecular Biology, 3rd edn (G. D. Fasman, ed.), Physical
and Chemical Data, Vol. 1, p. 122, CRC Press, 1976.
SThis is the value for free FAD; FAD bound to a specific flavoprotein (e.g. succinate dehydrogenase) has a ditferent E" that depends
on its protein environment.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:
9781305081079
Author:
STOKER, H. Stephen (howard Stephen)
Publisher:
Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning