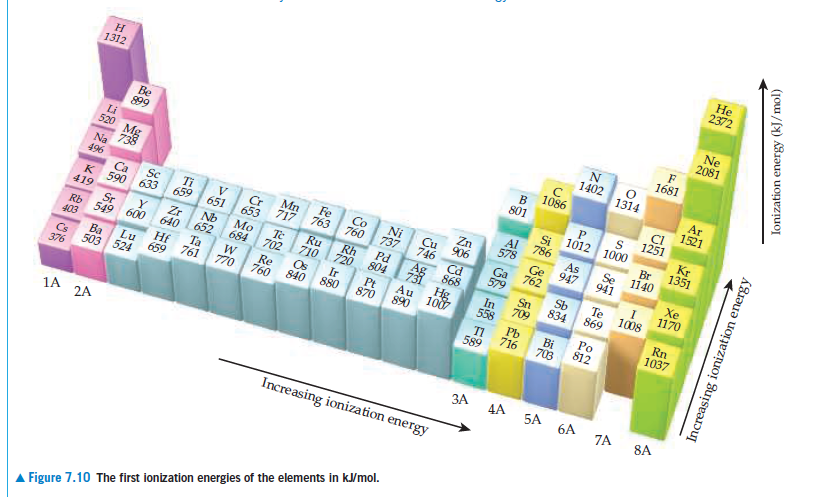
Не 2372 Ne 2081 н 1312 1681 1402 1314 Be 899 Ar 1521 1086 CI 1251 801 Li 520 Mg 738 1012 1000 Kr 1351 Na 496 Br 1140 786 As 947 Se 941 Zn 906 746 Cd * 868 578 Co Fe 760 Ni Sc Ti Ca Cr 653 717 763 Xe 1170 659 K 590 633 651 419 Rh 720 Pd 804 Ru 710 Te Sb 834 Nb Mo 684 Zr Te 702 Os 840 1008 Sr Sn 709 869 652 640 Rb 549 600 Rn 1037 Ir 880 Pt 870 Au 890 403 Ta 761 1007 558 Re 760 Hf 659 Po 812 Lu 770 Pb 716 Ba Bi 703 Cs 376 TI 589 503 524 1A 2A ЗА 4A 5A 6A 7A 8A Increasing ionization energy A Figure 7.10 The first ionization energies of the elements in kJ/mol. Increasing ionization energy Ionization energy (kJ/mol)
One way to measure ionization energies is ultraviolet photoelectron
spectroscopy (PES), a technique based on the
photoelectric effect. In PES, monochromatic
light is directed onto a sample, causing electrons to
be emitted. The kinetic energy of the emitted electrons is
measured. The difference between the energy of the photons
and the kinetic energy of the electrons corresponds to the
energy needed to remove the electrons (that is, the ionization
energy). Suppose that a PES experiment is performed
in which mercury vapor is irradiated with ultraviolet light
of wavelength 58.4 nm. (a) What is the energy of a photon
of this light, in joules? (b) Write an equation that shows the
process corresponding to the first ionization energy of Hg.
(c) The kinetic energy of the emitted electrons is measured to
be 1.72 x10-18 J. What is the first ionization energy of Hg, in kJ/mol? (d) Using Figure 7.10, determine which of the halogen
elements has a first ionization energy closest to that
of mercury.
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 10 images


