next question The following evidence was obtained during the titration of chromium (1) nitrate with an acidic potassium permanganate solution. Volume of chromium (II) nitrate samples -10.00 mL Concentration of KMNOA(ag)=0.0782 mol/L Volume of KMNO4(an) Trial 2. 3. Final buret reading (mL) 9.60 18.25 26.85 35.80 Initial buret reading (mL) 1.00 9.60 18.25 26.85 Final colour of mixture INink Pink Pink Pink 9. The concentration of the chromium () pits
next question The following evidence was obtained during the titration of chromium (1) nitrate with an acidic potassium permanganate solution. Volume of chromium (II) nitrate samples -10.00 mL Concentration of KMNOA(ag)=0.0782 mol/L Volume of KMNO4(an) Trial 2. 3. Final buret reading (mL) 9.60 18.25 26.85 35.80 Initial buret reading (mL) 1.00 9.60 18.25 26.85 Final colour of mixture INink Pink Pink Pink 9. The concentration of the chromium () pits
Chapter31: Introduction To Analytical Separations
Section: Chapter Questions
Problem 31.21QAP
Related questions
Question
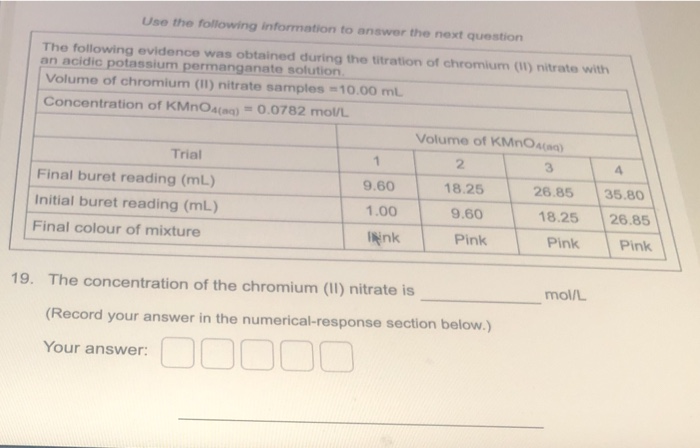
Transcribed Image Text:Use the following information to answer the next question
The following evidence was obtained during the titration of chromium (1) nitrate with
an acidic potassium permanganate solution.
Volume of chromium (II) nitrate samples =10.00 mL
Concentration of KMNOA(ag) = 0.0782 mol/L
Volume of KMnOA(aq)
Trial
2
4
Final buret reading (mL)
9.60
18.25
26.85
35.80
Initial buret reading (mL)
1.00
9.60
18.25
26.85
Final colour of mixture
INink
Pink
Pink
Pink
19. The concentration of the chromium (II) nitrate is
mol/L
(Record your answer in the numerical-response section below.)
Your answer: O0 000
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



