Nitric acid is a key industrial chemical, largely used to make fertilizers and explosives. The first step in its synthesis is the oxidation of ammonia. In this reaction, gaseous ammonia reacts with dioxygen gas to produce nitrogen monoxide gas and water. Suppose a chemical engineer studying a new catalyst for the oxidation of ammonia reaction finds that 990. liters per second of dioxygen are consumed when the reaction is run at 271. °C and the dioxygen is supplied at 0.30 atm. Calculate the rate at which nitrogen monoxide is being produced. Give your answer in kilograms per second. Be sure your answer has the correct number of significant digits. do kg
Nitric acid is a key industrial chemical, largely used to make fertilizers and explosives. The first step in its synthesis is the oxidation of ammonia. In this reaction, gaseous ammonia reacts with dioxygen gas to produce nitrogen monoxide gas and water. Suppose a chemical engineer studying a new catalyst for the oxidation of ammonia reaction finds that 990. liters per second of dioxygen are consumed when the reaction is run at 271. °C and the dioxygen is supplied at 0.30 atm. Calculate the rate at which nitrogen monoxide is being produced. Give your answer in kilograms per second. Be sure your answer has the correct number of significant digits. do kg
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter5: Gases
Section: Chapter Questions
Problem 5.98PAE
Related questions
Question
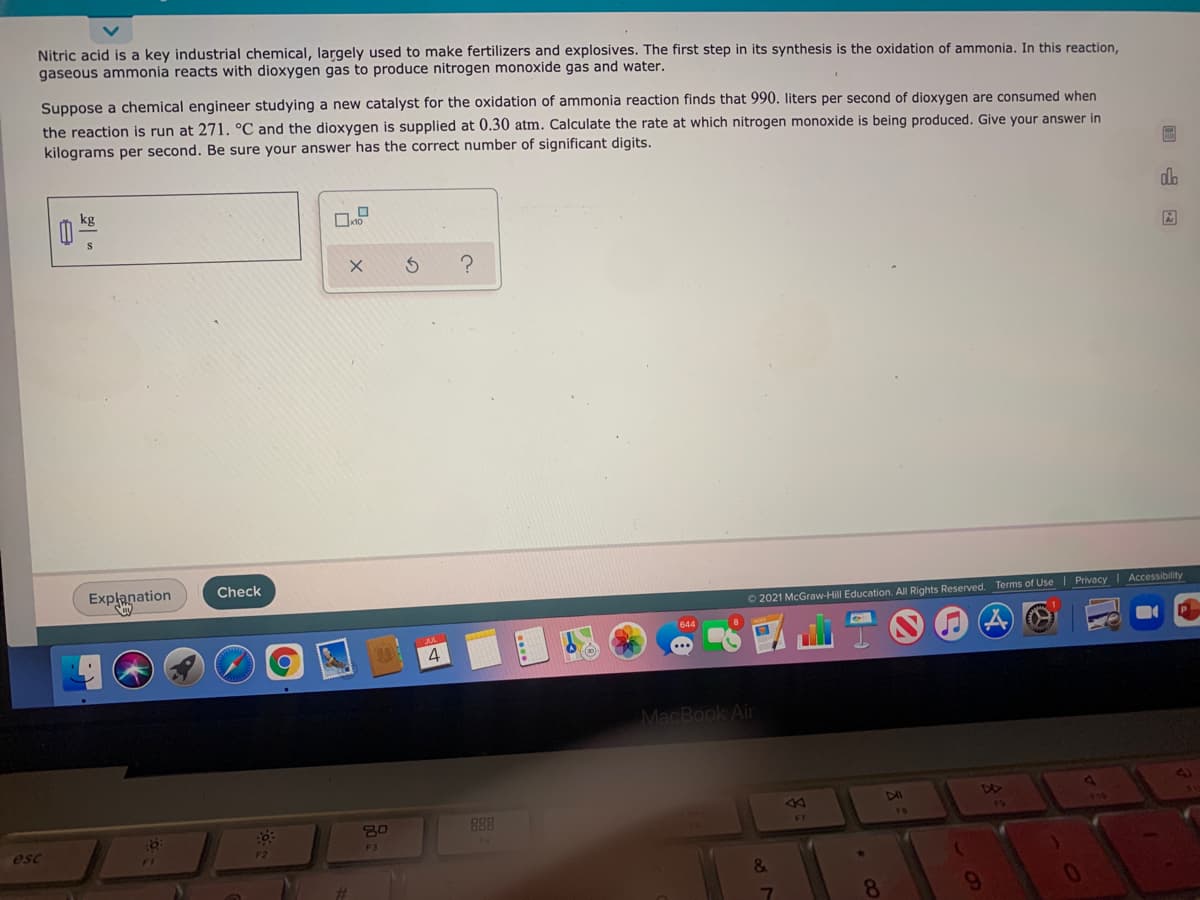
Transcribed Image Text:Nitric acid is a key industrial chemical, largely used to make fertilizers and explosives. The first step in its synthesis is the oxidation of ammonia. In this reaction,
gaseous ammonia reacts with dioxygen gas to produce nitrogen monoxide gas and water.
Suppose a chemical engineer studying a new catalyst for the oxidation of ammonia reaction finds that 990. liters per second of dioxygen are consumed when
the reaction is run at 271. °C and the dioxygen is supplied at 0.30 atm. Calculate the rate at which nitrogen monoxide is being produced. Give your answer in
kilograms per second. Be sure your answer has the correct number of significant digits.
kg
do
Explanation
Check
Accessibility
O 2021 McGraw-Hill Education. All Rights Reserved. Terms of Use | Privacy
644
4
MacBook Air
10
48
888
17
F7
FA
esc
F3
&.
8.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning