nitrobenzene You do not have to consider stereochemistry. • Include all valence lone pairs in your answer. • In cases where there is more than one answer, just draw one.
nitrobenzene You do not have to consider stereochemistry. • Include all valence lone pairs in your answer. • In cases where there is more than one answer, just draw one.
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter12: Chirality
Section: Chapter Questions
Problem 24CTQ
Related questions
Question
Draw a resonance structure, complete with all formal charges and lone (unshared) electron pairs, that shows the resonance interaction of the nitro with the para position in nitrobenzene.
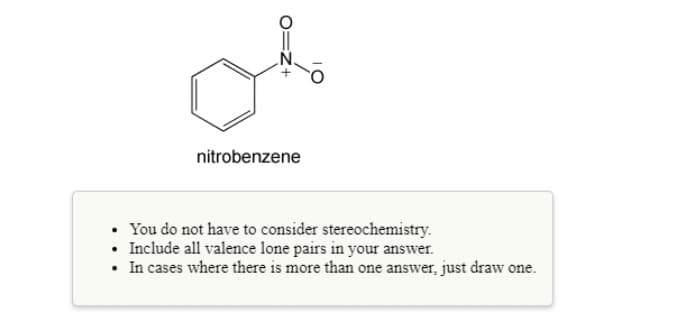
Transcribed Image Text:nitrobenzene
You do not have to consider stereochemistry.
• Include all valence lone pairs in your answer.
• In cases where there is more than one answer, just draw one.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning