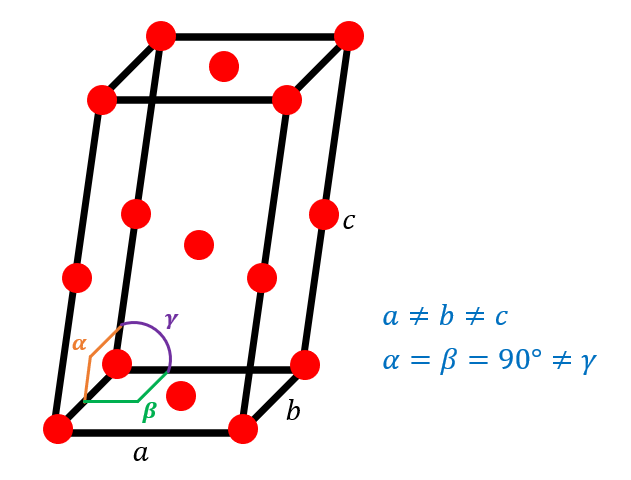
Not all unit cells have a cubic structure. Take for example the below image wherein the unit cell takes the shape of a parallelepiped and has lengths a, b, c and angles α, β, γ. This particular unit cell has an atom arrangement where it has one atom at each corner, one central atom at each of the bases, one atom at the center of the unit cell, and one atom at the center of each of the edge lengths across length c. Given a density of 25.26 g/cm3, calculate the length of edge c in angstroms of a theoretical element Ro given the following unit cell dimensions in angstroms and degrees: a = 9.6A b = 6.9A γ = 43o Atomic Mass: Ro: 48.844 g/mol
Not all unit cells have a cubic structure. Take for example the below image wherein the unit cell takes the shape of a parallelepiped and has lengths a, b, c and angles α, β, γ. This particular unit cell has an atom arrangement where it has one atom at each corner, one central atom at each of the bases, one atom at the center of the unit cell, and one atom at the center of each of the edge lengths across length c. Given a density of 25.26 g/cm3, calculate the length of edge c in angstroms of a theoretical element Ro given the following unit cell dimensions in angstroms and degrees: a = 9.6A b = 6.9A γ = 43o Atomic Mass: Ro: 48.844 g/mol
Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter31: Immunochemistry
Section: Chapter Questions
Problem 31.75P
Related questions
Question
Not all unit cells have a cubic structure. Take for example the below image wherein the unit cell takes the shape of a parallelepiped and has lengths a, b, c and angles α, β, γ. This particular unit cell has an atom arrangement where it has one atom at each corner, one central atom at each of the bases, one atom at the center of the unit cell, and one atom at the center of each of the edge lengths across length c. Given a density of 25.26 g/cm3, calculate the length of edge c in angstroms of a theoretical element Ro given the following unit cell dimensions in angstroms and degrees:
a = 9.6A
b = 6.9A
γ = 43o
Atomic Mass:
Ro: 48.844 g/mol
Transcribed Image Text:a + b # c
la
a = B = 90° + y
9.
a
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning



Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

