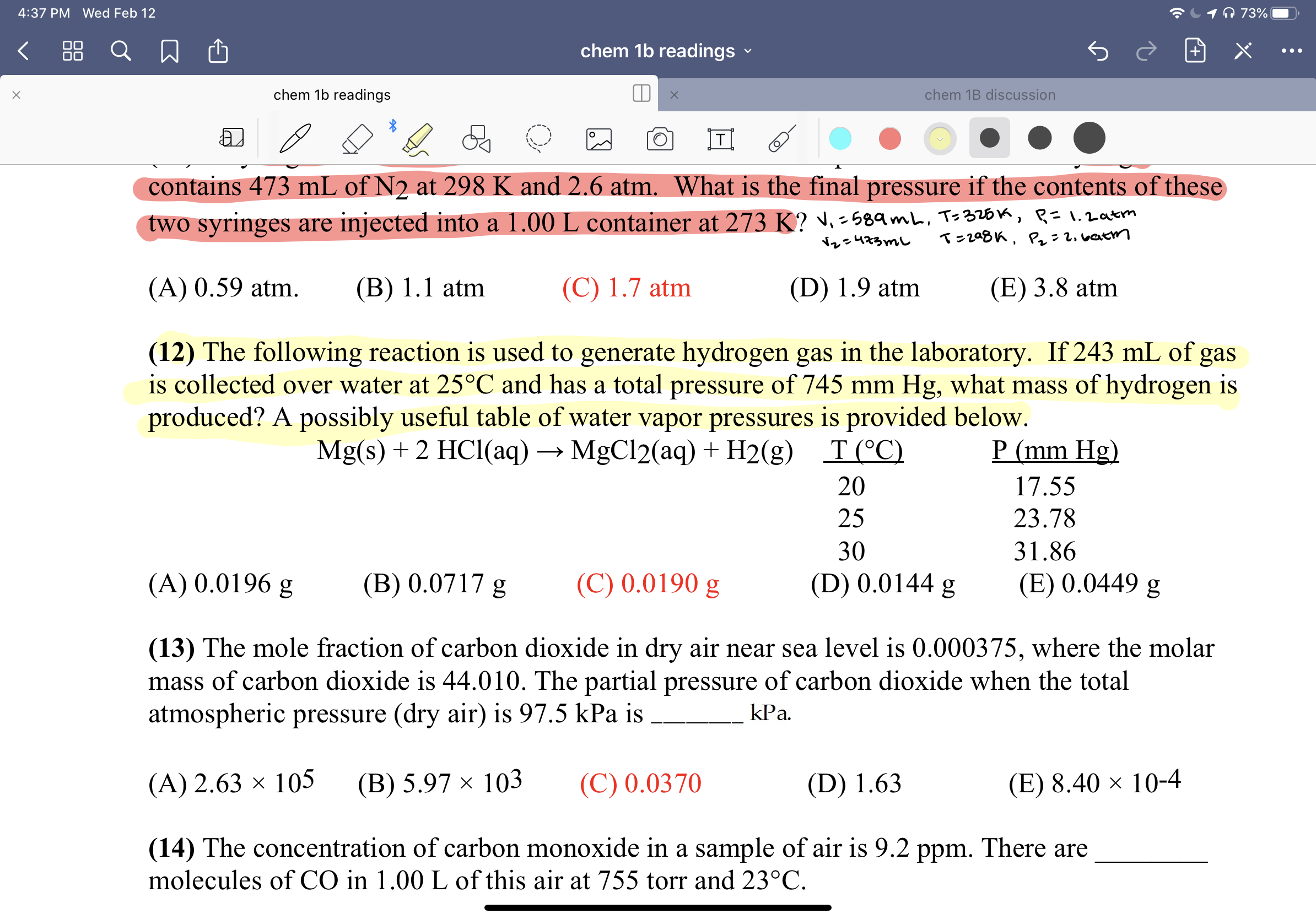
O 73% 4:37 PM Wed Feb 12 88 chem 1b readings chem 1b readings chem 1B discussion contains 473 mL of N2 at 298 K and 2.6 atm. What is the final pressure if the contents of these two syringes are injected into a 1.00 L container at 273 K? ♥. -589mL, Ts326k, R=1.2atm T=208K, P: 2. vatm Nレe473mし (A) 0.59 atm. (B) 1.1 atm (C) 1.7 atm (D) 1.9 atm (E) 3.8 atm (12) The following reaction is used to generate hydrogen gas in the laboratory. If 243 mL of gas is collected over water at 25°C and has a total pressure of 745 mm Hg, what mass of hydrogen is produced? A possibly useful table of water vapor pressures is provided below. P (mm Hg) Mg(s) + 2 HCl(aq) → MgCl2(aq) + H2(g) T(°C) 20 17.55 25 23.78 30 31.86 (C) 0.0190 g (A) 0.0196 g (B) 0.0717 g (D) 0.0144 g (E) 0.0449 g (13) The mole fraction of carbon dioxide in dry air near sea level is 0.000375, where the molar mass of carbon dioxide is 44.010. The partial pressure of carbon dioxide when the total atmospheric pressure (dry air) is 97.5 kPa is kPa. (C) 0.0370 (A) 2.63 × 105 (В) 5.97 х 103 (D) 1.63 (E) 8.40 × 10-4 (14) The concentration of carbon monoxide in a sample of air is 9.2 ppm. There are molecules of CO in 1.00 L of this air at 755 torr and 23°C.
O 73% 4:37 PM Wed Feb 12 88 chem 1b readings chem 1b readings chem 1B discussion contains 473 mL of N2 at 298 K and 2.6 atm. What is the final pressure if the contents of these two syringes are injected into a 1.00 L container at 273 K? ♥. -589mL, Ts326k, R=1.2atm T=208K, P: 2. vatm Nレe473mし (A) 0.59 atm. (B) 1.1 atm (C) 1.7 atm (D) 1.9 atm (E) 3.8 atm (12) The following reaction is used to generate hydrogen gas in the laboratory. If 243 mL of gas is collected over water at 25°C and has a total pressure of 745 mm Hg, what mass of hydrogen is produced? A possibly useful table of water vapor pressures is provided below. P (mm Hg) Mg(s) + 2 HCl(aq) → MgCl2(aq) + H2(g) T(°C) 20 17.55 25 23.78 30 31.86 (C) 0.0190 g (A) 0.0196 g (B) 0.0717 g (D) 0.0144 g (E) 0.0449 g (13) The mole fraction of carbon dioxide in dry air near sea level is 0.000375, where the molar mass of carbon dioxide is 44.010. The partial pressure of carbon dioxide when the total atmospheric pressure (dry air) is 97.5 kPa is kPa. (C) 0.0370 (A) 2.63 × 105 (В) 5.97 х 103 (D) 1.63 (E) 8.40 × 10-4 (14) The concentration of carbon monoxide in a sample of air is 9.2 ppm. There are molecules of CO in 1.00 L of this air at 755 torr and 23°C.
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter5: Gases
Section: Chapter Questions
Problem 52QAP: Nitrogen gas can be obtained by decomposing ammonium nitrate at high temperatures. The nitrogen gas...
Related questions
Question
I'm unsure of how to complete the question I attached. It's the question highlighted in yellow.
Transcribed Image Text:O 73%
4:37 PM Wed Feb 12
88
chem 1b readings
chem 1b readings
chem 1B discussion
contains 473 mL of N2 at 298 K and 2.6 atm. What is the final pressure if the contents of these
two syringes are injected into a 1.00 L container at 273 K? ♥. -589mL, Ts326k, R=1.2atm
T=208K, P: 2. vatm
Nレe473mし
(A) 0.59 atm.
(B) 1.1 atm
(C) 1.7 atm
(D) 1.9 atm
(E) 3.8 atm
(12) The following reaction is used to generate hydrogen gas in the laboratory. If 243 mL of gas
is collected over water at 25°C and has a total pressure of 745 mm Hg, what mass of hydrogen is
produced? A possibly useful table of water vapor pressures is provided below.
P (mm Hg)
Mg(s) + 2 HCl(aq) → MgCl2(aq) + H2(g)
T(°C)
20
17.55
25
23.78
30
31.86
(C) 0.0190 g
(A) 0.0196 g
(B) 0.0717 g
(D) 0.0144 g
(E) 0.0449 g
(13) The mole fraction of carbon dioxide in dry air near sea level is 0.000375, where the molar
mass of carbon dioxide is 44.010. The partial pressure of carbon dioxide when the total
atmospheric pressure (dry air) is 97.5 kPa is
kPa.
(C) 0.0370
(A) 2.63 × 105
(В) 5.97 х 103
(D) 1.63
(E) 8.40 × 10-4
(14) The concentration of carbon monoxide in a sample of air is 9.2 ppm. There are
molecules of CO in 1.00 L of this air at 755 torr and 23°C.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning