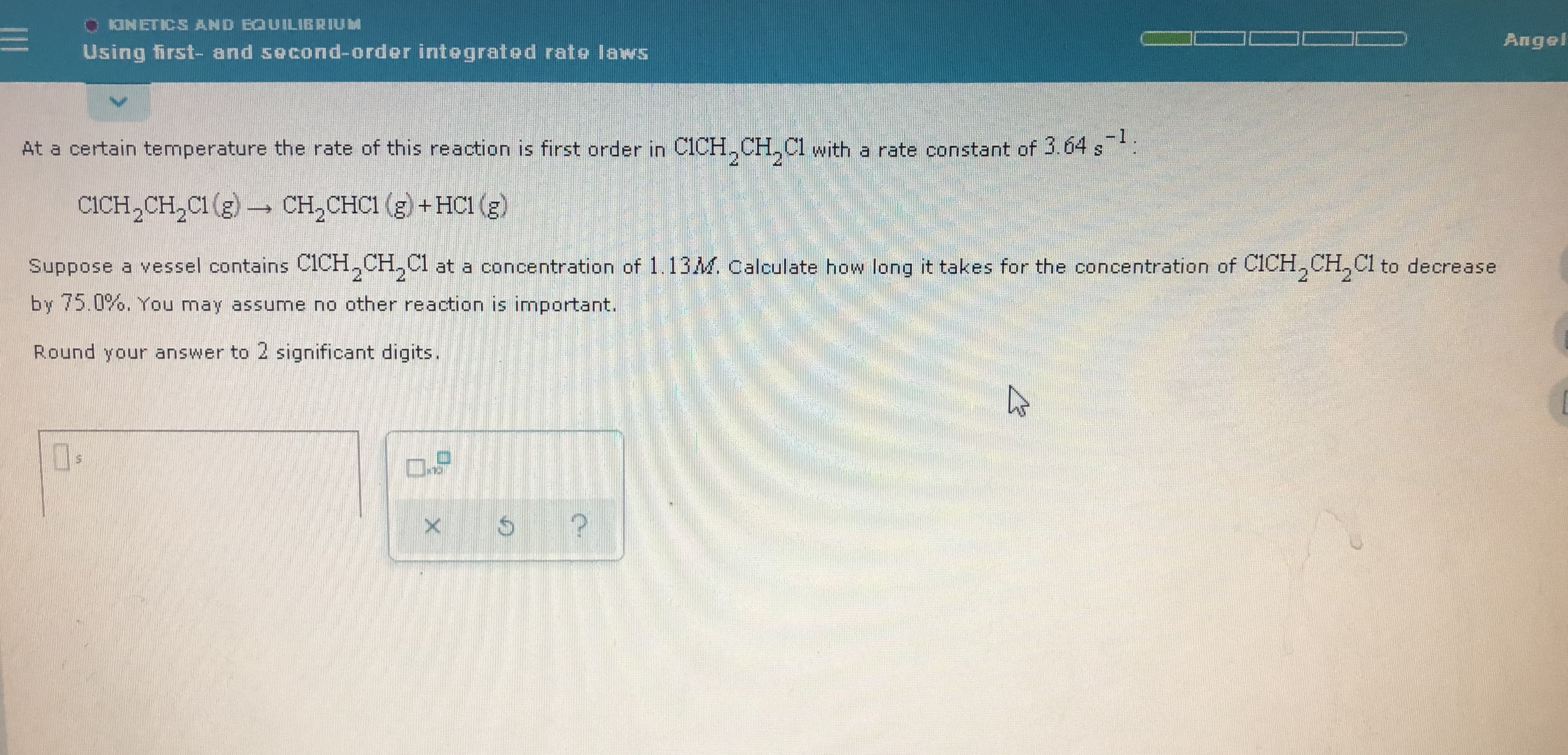
O ONETICS AND EQU ILIBRIUM Angel Using first- and second-order integrated rate laws -1 At a certain temperature the rate of this reaction is first order in C1CH,CH, Cl with a rate constant of 3.04 s: 2. CICH,CH,C1 (g) - CH,CHCI (g) + HC1 (g) Suppose a vessel contains CICH,CH,C1 at a concentration of 1.13M. Calculate how long it takes for the concentration of CICH,CH,C1 to decrease 2. by 75.0%. You may assume no other reaction is important. Round your answer to 2 significant digits.
O ONETICS AND EQU ILIBRIUM Angel Using first- and second-order integrated rate laws -1 At a certain temperature the rate of this reaction is first order in C1CH,CH, Cl with a rate constant of 3.04 s: 2. CICH,CH,C1 (g) - CH,CHCI (g) + HC1 (g) Suppose a vessel contains CICH,CH,C1 at a concentration of 1.13M. Calculate how long it takes for the concentration of CICH,CH,C1 to decrease 2. by 75.0%. You may assume no other reaction is important. Round your answer to 2 significant digits.
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter11: Chemical Kinetics: Rates Of Reactions
Section: Chapter Questions
Problem 116QRT
Related questions
Question
Transcribed Image Text:O ONETICS AND EQU ILIBRIUM
Angel
Using first- and second-order integrated rate laws
-1
At a certain temperature the rate of this reaction is first order in C1CH,CH, Cl with a rate constant of 3.04 s:
2.
CICH,CH,C1 (g) - CH,CHCI (g) + HC1 (g)
Suppose a vessel contains CICH,CH,C1 at a concentration of 1.13M. Calculate how
long it takes for the concentration of CICH,CH,C1 to decrease
2.
by 75.0%. You may assume no other reaction is important.
Round your answer to 2 significant digits.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning