Objectives: 1. Analyze sets of data and decide whether the measurements are precise and/or accurate. 2. Calculate percent error. The following sets of measurement for the density of a small cylinder of aluminum were given. The 'accepted' density of aluminium is 2.702 g/cm3. SET A SET B 2. 240 g/cm 2.700 g/cm3 2. 690 g/cm3 2.705 g/cm³ 2. 450 g/cm 2.703 g/cm 2. 150 g/cm3 2.701 g/cm3 1. Calculate the average value for cach set of density. 2. Calculate the % error for each set of values. 3. Compare the average value for each set with the accepted value: Which student's data is more accurate? Which student's data is more precise? 4. Compare the percentage error for each set: Which student's data is more accurate? Which student's data is more precise?
Objectives: 1. Analyze sets of data and decide whether the measurements are precise and/or accurate. 2. Calculate percent error. The following sets of measurement for the density of a small cylinder of aluminum were given. The 'accepted' density of aluminium is 2.702 g/cm3. SET A SET B 2. 240 g/cm 2.700 g/cm3 2. 690 g/cm3 2.705 g/cm³ 2. 450 g/cm 2.703 g/cm 2. 150 g/cm3 2.701 g/cm3 1. Calculate the average value for cach set of density. 2. Calculate the % error for each set of values. 3. Compare the average value for each set with the accepted value: Which student's data is more accurate? Which student's data is more precise? 4. Compare the percentage error for each set: Which student's data is more accurate? Which student's data is more precise?
Physics for Scientists and Engineers, Technology Update (No access codes included)
9th Edition
ISBN:9781305116399
Author:Raymond A. Serway, John W. Jewett
Publisher:Raymond A. Serway, John W. Jewett
Chapter1: Physics And Measurement
Section: Chapter Questions
Problem 1.61AP: The data in the following table represent measurements of the masses and dimensions of solid...
Related questions
Question
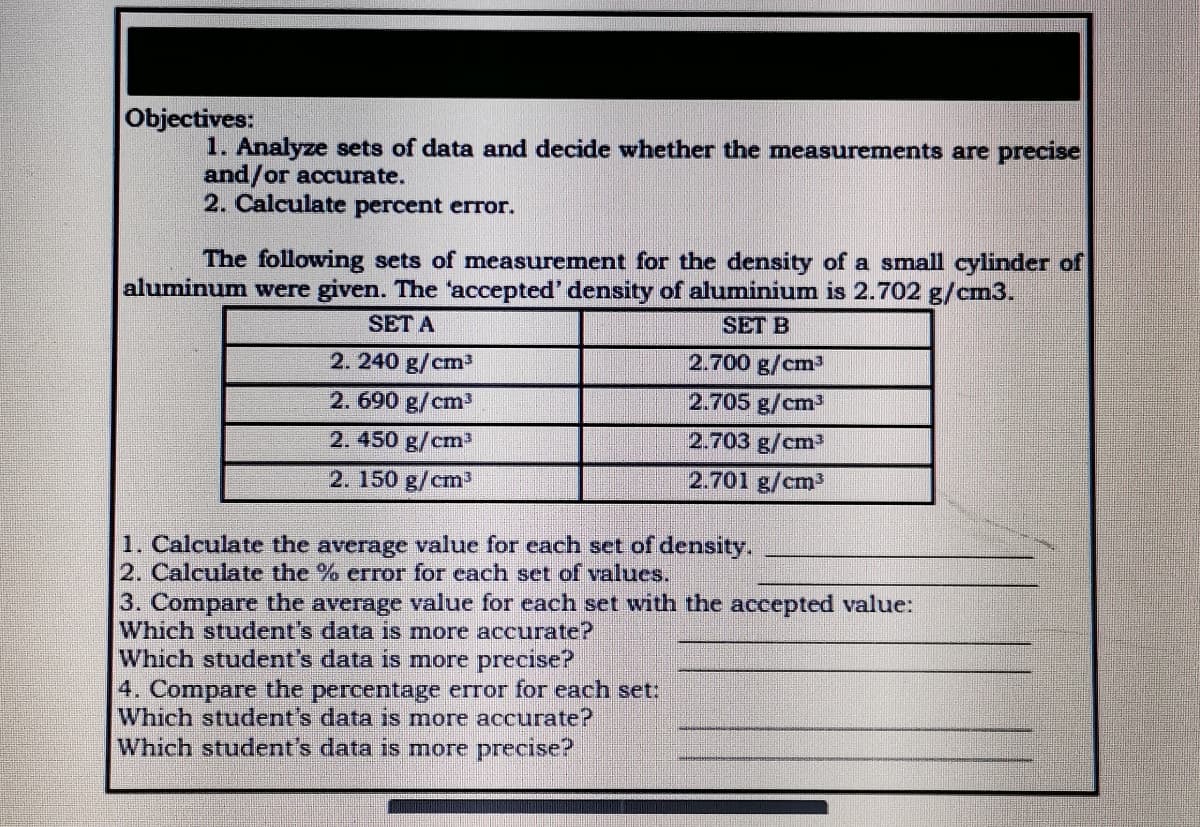
Transcribed Image Text:Objectives:
1. Analyze sets of data and decide whether the measurements are precise
and/or accurate.
2. Calculate percent error.
The following sets of measurement for the density of a small cylinder of
aluminum were given. The "accepted' density of aluminium is 2.702 g/cm3.
SET A
SET B
2. 240 g/cm
2.700 g/cm3
2.690 g/cm3
2.705 g/cm3
2.450 g/cm3
2.703 g/cm³
2. 150 g/cm
2.701 g/cm³
1. Calculate the average value for each set of density.
2. Calculate the % error for each set of values.
3. Compare the average value for each set with the accepted value:
Which student's data is more accurate?
Which student's data is more precise?
4. Compare the percentage error for each set:
Which student's data is more accurate?
Which student's data is more precise?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College

Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College

Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning