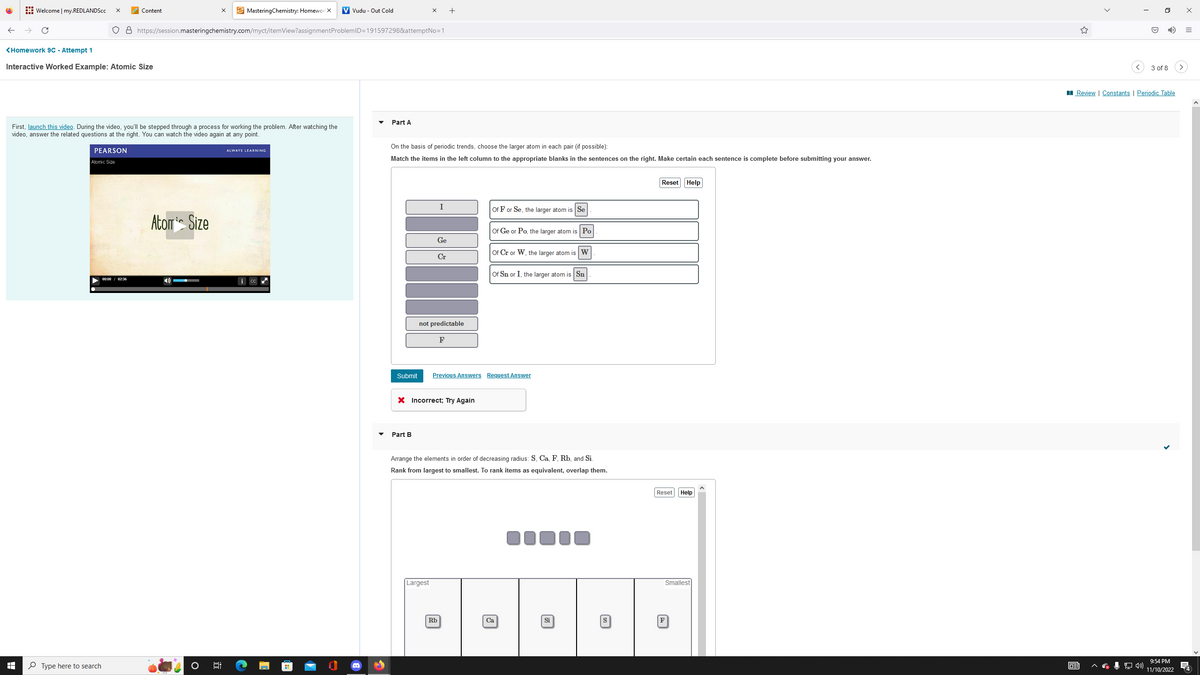
On the basis of periodic trends, choose the larger atom in each pair (f possible) Match the items in the left column to the appropriate blanks in the sentences on the right. Make certain each sentence is complete before submitting your answer. not predictable F of For Se, the larger atom is Se of Ge or Po, the larger atom is Po or Cror W, the larger atomi W or Sn or 1, the larger atom is So Reset Help
On the basis of periodic trends, choose the larger atom in each pair (f possible) Match the items in the left column to the appropriate blanks in the sentences on the right. Make certain each sentence is complete before submitting your answer. not predictable F of For Se, the larger atom is Se of Ge or Po, the larger atom is Po or Cror W, the larger atomi W or Sn or 1, the larger atom is So Reset Help
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.9QAP
Related questions
Question
How do I solve for part A of this chemitry question.
Transcribed Image Text:Welcome | my.REDLANDScc
I
MasteringChemistry: Homewor X V Vudu - Out Cold
O8 https://session.masteringchemistry.com/myct/itemView?assignment ProblemID=191597298&attemptNo=1
X
<Homework 9C - Attempt 1
Interactive Worked Example: Atomic Size
First, launch this video. During the video, you'll be stepped through a process for working the problem. After watching the
video, answer the related questions at the right. You can watch the video again at any point.
Type here to search
Content
PEARSON
Atomic Size
Atom Size
e
D
6:
20
ALWAYS LEARNING
Part A
Submit
On the basis of periodic trends, choose the larger atom in each pair (if possible):
Match the items in the left column to the appropriate blanks in the sentences on the right. Make certain each sentence is complete before submitting your answer.
X
Part B
Ge
Cr
+
not predictable
F
Largest
X Incorrect; Try Again
Of F or Se, the larger atom is Se
Rb
Of Ge or Po, the larger atom is Po
Of Cr or W, the larger atom is W
Previous Answers Request Answer
Of Sn or I, the larger atom is Sn
Arrange the elements in order of decreasing radius: S, Ca, F, Rb, and Si.
Rank from largest to smallest. To rank items as equivalent, overlap them.
Ca
☐☐☐☐
Si
S
Reset Help
Reset
Help
Smallest
<
GE
0
Review | Constants | Periodic Table
())
3 of 8
9:54 PM
11/10/2022
x
=
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

