One of the more famous species in coordination chemistry is the Creutz-Taube complex: 5+ (NH3);RuN NRu(NH3)5 It is named for the two scientists who discovered it and ini- tially studied its properties. The central ligand is pyrazine, a planar six-membered ring with nitrogens at opposite sides. (a) How can you account for the fact that the com- plex, which has only neutral ligands, has an odd overall charge? (b) The metal is in a low-spin configuration in both cases. Assuming octahedral coordination, draw the d-orbital energy-level diagram for each metal. (c) In many experiments the two metal ions appear to be in exactly equivalent states. Can you think of a reason that this might appear to be so, recognizing that electrons move very rap- idly compared to nuclel?
One of the more famous species in coordination chemistry is the Creutz-Taube complex: 5+ (NH3);RuN NRu(NH3)5 It is named for the two scientists who discovered it and ini- tially studied its properties. The central ligand is pyrazine, a planar six-membered ring with nitrogens at opposite sides. (a) How can you account for the fact that the com- plex, which has only neutral ligands, has an odd overall charge? (b) The metal is in a low-spin configuration in both cases. Assuming octahedral coordination, draw the d-orbital energy-level diagram for each metal. (c) In many experiments the two metal ions appear to be in exactly equivalent states. Can you think of a reason that this might appear to be so, recognizing that electrons move very rap- idly compared to nuclel?
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter20: Chemistry Of Selected Transition Elements And Coordination Compounds
Section: Chapter Questions
Problem 105QRT
Related questions
Question
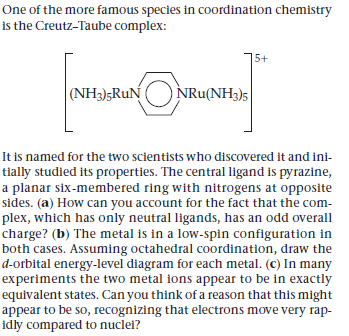
Transcribed Image Text:One of the more famous species in coordination chemistry
is the Creutz-Taube complex:
5+
(NH3);RuN
NRu(NH3)5
It is named for the two scientists who discovered it and ini-
tially studied its properties. The central ligand is pyrazine,
a planar six-membered ring with nitrogens at opposite
sides. (a) How can you account for the fact that the com-
plex, which has only neutral ligands, has an odd overall
charge? (b) The metal is in a low-spin configuration in
both cases. Assuming octahedral coordination, draw the
d-orbital energy-level diagram for each metal. (c) In many
experiments the two metal ions appear to be in exactly
equivalent states. Can you think of a reason that this might
appear to be so, recognizing that electrons move very rap-
idly compared to nuclel?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax