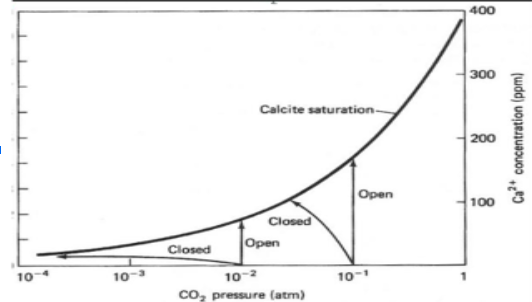
Open bodies of water can act as carbon sinks due to the ability of carbon dioxide to dissolve in water. The dissolution of carbon dioxide produces carbonic acid (H2CO3) which then partially dissociates in an equilibrium reaction important for controlling water pH. CO2 (g) + H2O (l) H2CO3 (aq) H2CO3 (aq) ↔ H+ (aq) + HCO3- (aq) The equilibrium between carbonic acid (H2CO3) and bicarbonate (HCO3-) is influenced by the presence of dissolved metals from the bedrock underlying a body of water. Calcium from limestone (a type of sedimentary rock formed from marine exoskeletons) is one such metal. The graph below shows how the concentration of dissolved calcium ions (measured in parts per million, or mg/L) affects the concentration of carbon dioxide dissolved in ocean water (measured partial pressure). Based on this graph and your understanding of equilibrium, in what direction does the presence of limestone shift the carbonic acid/bicarbonate equilibrium?
Open bodies of water can act as carbon sinks due to the ability of carbon dioxide to dissolve in water. The dissolution of carbon dioxide produces carbonic acid (H2CO3) which then partially dissociates in an equilibrium reaction important for controlling water pH.
CO2 (g) + H2O (l) H2CO3 (aq)
H2CO3 (aq) ↔ H+ (aq) + HCO3- (aq)
The equilibrium between carbonic acid (H2CO3) and bicarbonate (HCO3-) is influenced by the presence of dissolved metals from the bedrock underlying a body of water. Calcium from limestone (a type of sedimentary rock formed from marine exoskeletons) is one such metal. The graph below shows how the concentration of dissolved calcium ions (measured in parts per million, or mg/L) affects the concentration of carbon dioxide dissolved in ocean water (measured partial pressure). Based on this graph and your understanding of equilibrium, in what direction does the presence of limestone shift the carbonic acid/bicarbonate equilibrium?
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images


