Oxidation and reduction reactions are symbiotic (one can't exist without the other). Cite evidence from the battery simulation to support this truth.
Oxidation and reduction reactions are symbiotic (one can't exist without the other). Cite evidence from the battery simulation to support this truth.
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter21: Chemistry Of The Main-group Elements
Section: Chapter Questions
Problem 21.190QP
Related questions
Question
Use one example from the chart.
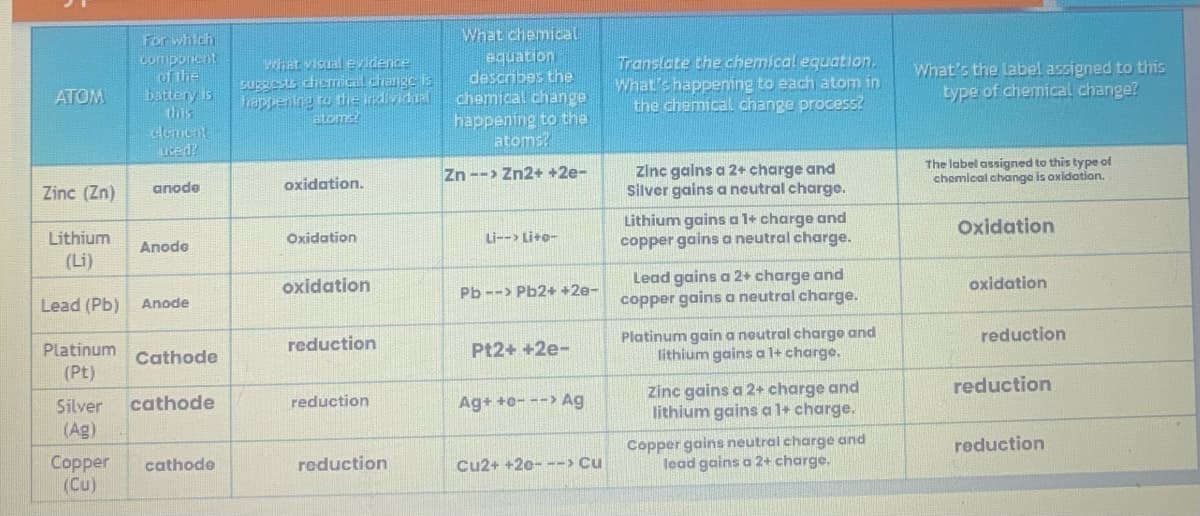
Transcribed Image Text:ATOM
Zinc (Zn)
Platinum
(Pt)
For which
component
of the
battery is
this
element
used?
Lithium
(Li)
Lead (Pb) Anode
anode
Copper
(Cu)
Anode
Cathode
Silver cathode
(Ag)
cathode
What visual evidence
suggests chemical change is
happening to the individual
atoms!
oxidation.
Oxidation
oxidation
reduction
reduction
reduction
What chemical
equation
describes the
chemical change
happening to the
atoms?
Zn --> Zn2+ +2e-
Li--> Li+o-
Pb--> Pb2+ +2e-
Pt2+ +2e-
Ag+ +0---> Ag
Cu2+ +20---> Cu
Translate the chemical equation.
What's happening to each atom in
the chemical change process?
zinc gains a 2+ charge and
Silver gains a neutral charge.
Lithium gains a 1+ charge and
copper gains a neutral charge.
Lead gains a 2+ charge and
copper gains a neutral charge.
Platinum gain a neutral charge and
lithium gains a 1+ charge.
zinc gains a 2+ charge and
lithium gains a 1+ charge.
Copper gains neutral charge and
lead gains a 2+ charge,
What's the label assigned to this
type of chemical change?
The label assigned to this type of
chemical change is oxidation.
Oxidation
oxidation
reduction
reduction
reduction

Transcribed Image Text:Oxidation and reduction reactions are symbiotic (one can't exist without the other).
Cite evidence from the battery simulation to support this truth.
Expert Solution
Step 1
- Oxidation is defined as the gain of an oxygen atom or the loss of electrons. Thus, during oxidation of a species, the oxidation state of that species increases.
- Reduction is defined as the loss of an oxygen atom or gain of a hydrogen atom or the gain of electrons. Thus, during the reduction of a species, the oxidation state of that species decreases.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co