Pacemakers are electronic devices that help regulate the heart rate. Currently, lithium-iodine cells are commonly used to power pacemakers and have replaced zinc-mercury cells. Table 1 provides the operating cell potential, E, for each cell. Table 2 provides the standard reduction potentials for several half-reactions related to zinc-mercury and zinc-air cells. The use of zinc-mercury cells in hearing aids has been replaced by zinc-air cells that operate using the oxidation of Zn by O2 from the air, generating a potential of +1.60 V. Table 2 provides the standard reduction potentials for the half-reactions used in zinc-mercury and zinc-air cells. Which of the following best explains the modification to the cell design that is mostly responsible for the difference in standard cell potentials for zinc-mercury and zinc-air cells? The greater standard cell potential of the Zn-air cell compared to that of the zinc-mercury cell most likely results from the thermodynamically more favorable reduction of Oz compared to A HgO. The greater standard cell potential of the Zn-air cell compared to that of the zinc-mercury cell most likely results from the greater number of moles of e required to reduce Oz compared to B HgO. The greater standard cell potential of the Zn-air cell compared to that of the zinc-mercury cell most likely results from the thermodynamically less favorable reduction of Oz compared to HgO. The greater standard cell potential of the Zn-air cell compared to that of the zinc-mercury cell most likely results from the greater number of moles of hydroxide ions required to reduce D Zn(OH), compared to Zn(OH),-
Pacemakers are electronic devices that help regulate the heart rate. Currently, lithium-iodine cells are commonly used to power pacemakers and have replaced zinc-mercury cells. Table 1 provides the operating cell potential, E, for each cell. Table 2 provides the standard reduction potentials for several half-reactions related to zinc-mercury and zinc-air cells. The use of zinc-mercury cells in hearing aids has been replaced by zinc-air cells that operate using the oxidation of Zn by O2 from the air, generating a potential of +1.60 V. Table 2 provides the standard reduction potentials for the half-reactions used in zinc-mercury and zinc-air cells. Which of the following best explains the modification to the cell design that is mostly responsible for the difference in standard cell potentials for zinc-mercury and zinc-air cells? The greater standard cell potential of the Zn-air cell compared to that of the zinc-mercury cell most likely results from the thermodynamically more favorable reduction of Oz compared to A HgO. The greater standard cell potential of the Zn-air cell compared to that of the zinc-mercury cell most likely results from the greater number of moles of e required to reduce Oz compared to B HgO. The greater standard cell potential of the Zn-air cell compared to that of the zinc-mercury cell most likely results from the thermodynamically less favorable reduction of Oz compared to HgO. The greater standard cell potential of the Zn-air cell compared to that of the zinc-mercury cell most likely results from the greater number of moles of hydroxide ions required to reduce D Zn(OH), compared to Zn(OH),-
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter25: Voltammetry
Section: Chapter Questions
Problem 25.10QAP: Quinone undergoes a reversible reduction at a voltammetric working electrode. The reaction is (a)...
Related questions
Question
Transcribed Image Text:CollegeBoard
AP Classroom
Unit 9 Progress Check: MCQ
Ebraam Awad
11
12
13
27
« < 2 of 30
Zinc-mercury
+1.35
Table 2
Half-Reaction
Standard Reduction Potential, E (V)
[Zn(OH),J- + 2 e Zn + 4 OH
Zn(OH), + 2e- → Zn + 2 OH
-1.20
-1.25
Hgo + H20 + 2 e → Hg + 2OH
+0.10
O2 + 2 H20 + 4e 4 OH
+0.40
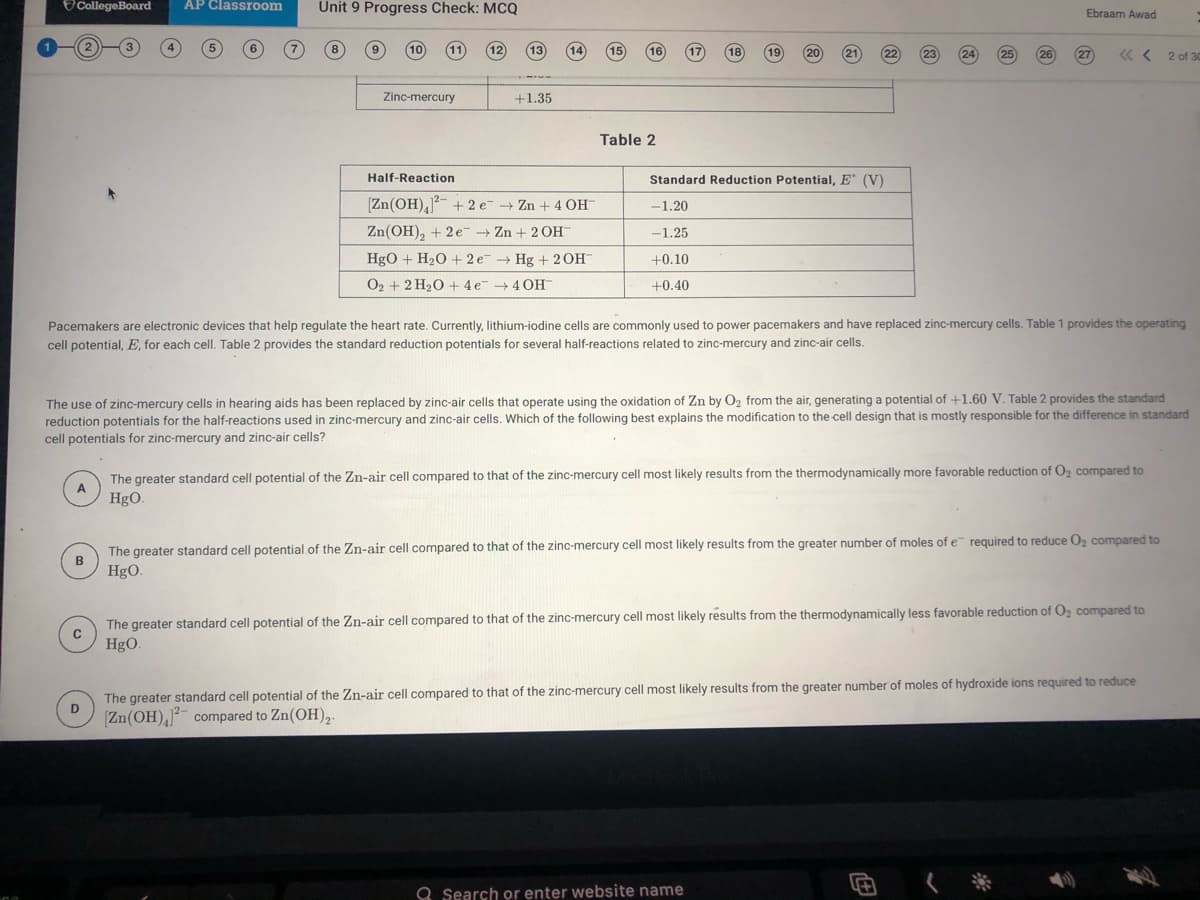
Pacemakers are electronic devices that help regulate the heart rate. Currently, lithium-iodine cells are commonly used to power pacemakers and have replaced zinc-mercury cells. Table 1 provides the operating
cell potential, E, for each cell. Table 2 provides the standard reduction potentials for several half-reactions related to zinc-mercury and zinc-air cells.
The use of zinc-mercury cells in hearing aids has been replaced by zinc-air cells that operate using the oxidation of Zn by O2 from the air, generating a potential of +1.60 V. Table 2 provides the standard
reduction potentials for the half-reactions used in zinc-mercury and zinc-
cell potentials for zinc-mercury and zinc-air cells?
cells. Which of the following best explains the modification to the cell design that is mostly responsible for the difference in standard
The greater standard cell potential of the Zn-air cell compared to that of the zinc-mercury cell most likely results from the thermodynamically more favorable reduction of O2 compared to
A
HgO.
The greater standard cell potential of the Zn-air cell compared to that of the zinc-mercury cell most likely results from the greater number of moles of e required to reduce Oz compared to
B
HgO.
The greater standard cell potential of the Zn-air cell compared to that of the zinc-mercury cell most likely results from the thermodynamically less favorable reduction of O, compared to
HgO.
The greater standard cell potential of the Zn-air cell compared to that of the zinc-mercury cell most likely results from the greater number of moles of hydroxide ions required to reduce
D
Zn(OH),- compared to Zn(OH),-
Q Search or enter website name
Transcribed Image Text:A apclassroom.collegeboard.org
CollegeBoard
AP Classroom
Unit 9 Progress Check: MCQ
Ebraam Awad
9
12
13
14
(16
21
« <
2 of 30 > >»>
Table 1
Cell Type
Operating Cell Potential for Commercial Batteries, E (V)
Lithium-iodine
+2.80
Zinc-mercury
+1.35
Table 2
Submit
Half-Reaction
Standard Reduction Potential, E (V)
[Zn(OH),- + 2 e Zn + 4 OH
-1.20
Zn(OH), + 2e- + Zn + 2 OH
HgO + H20 + 2 e Hg + 2OH
-1.25
+0.10
O2 + 2 H2O + 4 e +4 OH
+0.40
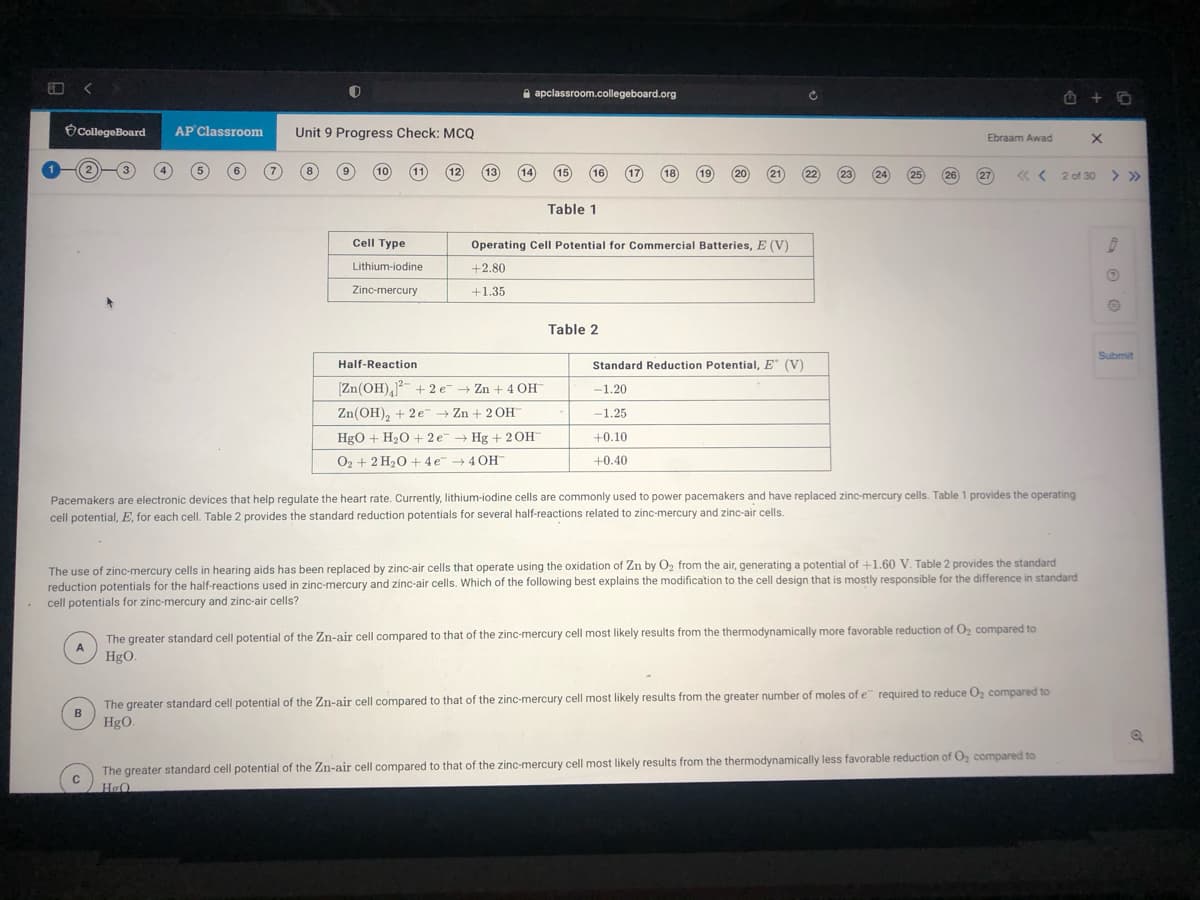
Pacemakers are electronic devices that help regulate the heart rate. Currently, lithium-iodine cells are commonly used to power pacemakers and have replaced zinc-mercury cells. Table 1 provides the operating
cell potential, E, for each cell. Table 2 provides the standard reduction potentials for several half-reactions related to zinc-mercury and zinc-air cells.
The use of zinc-mercury cells in hearing aids has been replaced by zinc-air cells that operate using the oxidation of Zn by Oz from the air, generating a potential of +1.60 V. Table 2 provides the standard
reduction potentials for the half-reactions used in zinc-mercury and zinc-air cells. Which of the following best explains the modification to the cell design that is mostly responsible for the difference in standard
cell potentials for zinc-mercury and zinc-air cells?
The greater standard cell potential of the Zn-air cell compared to that of the zinc-mercury cell most likely results from the thermodynamically more favorable reduction of O, compared to
HgO.
The greater standard cell potential of the Zn-air cell compared to that of the zinc-mercury cell most likely results from the greater number of moles of e required to reduce O, compared to
B
HgO.
The greater standard cell potential of the Zn-air cell compared to that of the zinc-mercury cell most likely results from the thermodynamically less favorable reduction of O, compared to
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning
