Para-aminobenzoic acid has two ionizable groups. One is a carboxylic acid that ionizes through loss of a proton thereby forming a charged para-aminobenzoic acid carboxylate anion, and the other is an amine connected to an aromatic (benzene) ring that ionizes by accepting an H+ to form a positively charged ammonium ion. The pk of the carboxylic acid is 4.9 and the pk of the amino group is 2.5. Assume that the intracellular pH within a bacterial cell is 7.0. H H O-H pk = 2.5 Which of the following structures correctly depicts how most of the para-aminobenzoic acid is ionized at pH 7? A. B. H + H-N- H C. H H. + H-N- H N- H O-H pK₂ = 4.9 D. X o o O-H
Para-aminobenzoic acid has two ionizable groups. One is a carboxylic acid that ionizes through loss of a proton thereby forming a charged para-aminobenzoic acid carboxylate anion, and the other is an amine connected to an aromatic (benzene) ring that ionizes by accepting an H+ to form a positively charged ammonium ion. The pk of the carboxylic acid is 4.9 and the pk of the amino group is 2.5. Assume that the intracellular pH within a bacterial cell is 7.0. H H O-H pk = 2.5 Which of the following structures correctly depicts how most of the para-aminobenzoic acid is ionized at pH 7? A. B. H + H-N- H C. H H. + H-N- H N- H O-H pK₂ = 4.9 D. X o o O-H
Chapter26: Biomolecules: Amino Acids, Peptides, And Proteins
Section26.SE: Something Extra
Problem 50AP: The -helical parts of myoglobin and other proteins stop whenever a proline residue is encountered in...
Related questions
Question
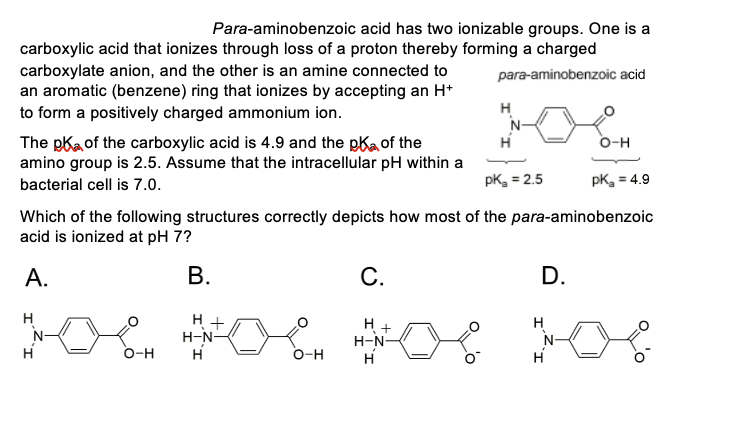
Transcribed Image Text:Para-aminobenzoic acid has two ionizable groups. One is a
carboxylic acid that ionizes through loss of a proton thereby forming a charged
para-aminobenzoic acid
carboxylate anion, and the other is an amine connected to
an aromatic (benzene) ring that ionizes by accepting an H+
to form a positively charged ammonium ion.
The pk of the carboxylic acid is 4.9 and the pk of the
amino group is 2.5. Assume that the intracellular pH within a
bacterial cell is 7.0.
N-
H
O-H
H +
H-N-
H
pK₂ = 2.5
Which of the following structures correctly depicts how most of the para-aminobenzoic
acid is ionized at pH 7?
A.
B.
O-H
C.
H
H +
H-N-
H
H
D.
H
N-
O-H
H
pK₂ = 4.9
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning