• Part A Enter a balanced reaction for the complete combustion of hydrogen. Express your answer as a chemical equation. Identify all of the phases in your answer. 2H2(g)+ O2(g)+2H20 (g) Submit Previous Answers v Correct The balanced combustion equation is 2H2(g) + O2(g)–→2H2O(g) • Part B Use standard enthalpies of formation to calculate the amount of heat released per kilogram of hydrogen fuel. Express your answer using four significant figures. amount of heat released = 1.418×105 kJ/kg Previous Answers Submit All attempts used; correct answer displayed The enthalpy of formation of water can be used to calculate the amount of energy released per kilogram of fuel. Since only water is present in a nonstandard state, the enthalpy for the reaction is equal to the enthalpy of formation of water. Thus 1 mol 0.002016 kg AH H20 (kJ/mol) × where 0.002016 kg/mol is the molar mass of hydrogen gas. • Part C Enter a balanced reaction for the complete combustion of methanol. Express your answer as a chemical equation. Identify all of the phases in your answer. 2CH3OH(1)+ 302(g}>2CO2(g)+ 4H20(g) Submit Previous Answers v Correct The balanced combustion equation is 2CH3OH(1) + 302(g) → 2CO2(g) + 4H20(g) , Part D Use standard enthalpies of formation to calculate the amount of heat released per kilogram of methanol fuel. Express your answer using four significant figures. amount of heat released = 2,267x104 kJ/kg Submit Previous Answers v Correct The enthalpies of formation of carbon dioxide, water, and methanol can be used to calculate the amount of energy released per kilogram of fuel as follows: AHfxn = npA Hf(products) – nrA Hf(reactants) [(AH? CO2 + 2(A HỆ H20 ) – AH? CH3OH] (kJ/mol) Once you have the enthalpy change for the reaction, divide by the molar mass of the fuel, methanol, in kg/mol to find the heat released. , Part E Which fuel contains the most energy in the least mass? hydrogen methanol Previous Answers Submit v Correct Using the results from Parts B and D of the question. Hydrogen contributes almost an order of magnitude more energy per kilogram of mass than does methanol. • Part F To compare the energy of these fuels to that of octane (CgH19 , calculate the energy released per kilogram of octane. Express your answer using four significant figures. kJ/kg amount of heat released by octane =
• Part A Enter a balanced reaction for the complete combustion of hydrogen. Express your answer as a chemical equation. Identify all of the phases in your answer. 2H2(g)+ O2(g)+2H20 (g) Submit Previous Answers v Correct The balanced combustion equation is 2H2(g) + O2(g)–→2H2O(g) • Part B Use standard enthalpies of formation to calculate the amount of heat released per kilogram of hydrogen fuel. Express your answer using four significant figures. amount of heat released = 1.418×105 kJ/kg Previous Answers Submit All attempts used; correct answer displayed The enthalpy of formation of water can be used to calculate the amount of energy released per kilogram of fuel. Since only water is present in a nonstandard state, the enthalpy for the reaction is equal to the enthalpy of formation of water. Thus 1 mol 0.002016 kg AH H20 (kJ/mol) × where 0.002016 kg/mol is the molar mass of hydrogen gas. • Part C Enter a balanced reaction for the complete combustion of methanol. Express your answer as a chemical equation. Identify all of the phases in your answer. 2CH3OH(1)+ 302(g}>2CO2(g)+ 4H20(g) Submit Previous Answers v Correct The balanced combustion equation is 2CH3OH(1) + 302(g) → 2CO2(g) + 4H20(g) , Part D Use standard enthalpies of formation to calculate the amount of heat released per kilogram of methanol fuel. Express your answer using four significant figures. amount of heat released = 2,267x104 kJ/kg Submit Previous Answers v Correct The enthalpies of formation of carbon dioxide, water, and methanol can be used to calculate the amount of energy released per kilogram of fuel as follows: AHfxn = npA Hf(products) – nrA Hf(reactants) [(AH? CO2 + 2(A HỆ H20 ) – AH? CH3OH] (kJ/mol) Once you have the enthalpy change for the reaction, divide by the molar mass of the fuel, methanol, in kg/mol to find the heat released. , Part E Which fuel contains the most energy in the least mass? hydrogen methanol Previous Answers Submit v Correct Using the results from Parts B and D of the question. Hydrogen contributes almost an order of magnitude more energy per kilogram of mass than does methanol. • Part F To compare the energy of these fuels to that of octane (CgH19 , calculate the energy released per kilogram of octane. Express your answer using four significant figures. kJ/kg amount of heat released by octane =
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter9: Energy And Chemistry
Section: Chapter Questions
Problem 9.102PAE: 9.102 A runner generates 418 kJ of energy per kilometer from the cellular oxidation of food. The...
Related questions
Question
please answer part f using the provided information in the images
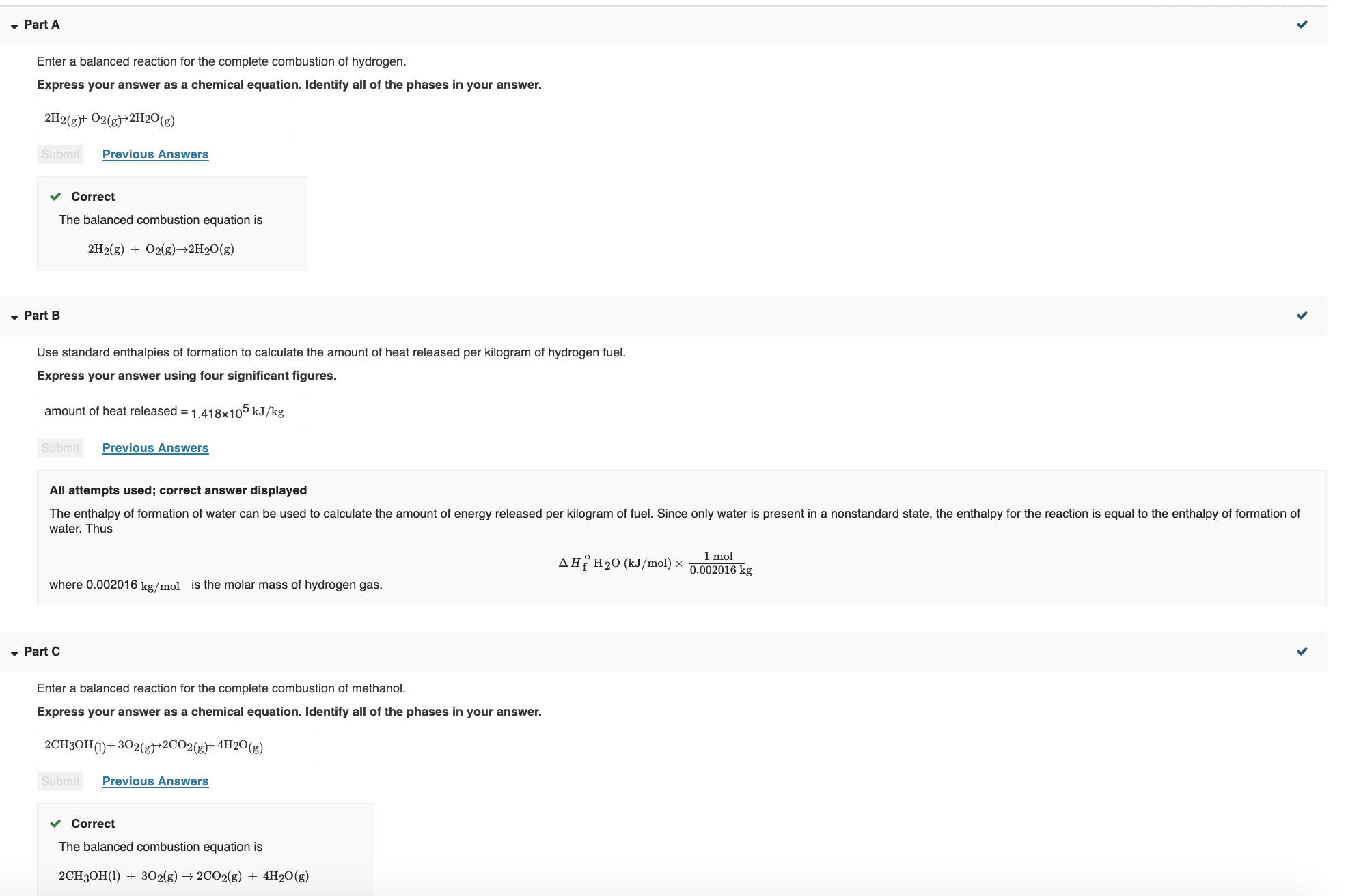
Transcribed Image Text:• Part A
Enter a balanced reaction for the complete combustion of hydrogen.
Express your answer as a chemical equation. Identify all of the phases in your answer.
2H2(g)+ O2(g)+2H20 (g)
Submit
Previous Answers
v Correct
The balanced combustion equation is
2H2(g) + O2(g)–→2H2O(g)
• Part B
Use standard enthalpies of formation to calculate the amount of heat released per kilogram of hydrogen fuel.
Express your answer using four significant figures.
amount of heat released = 1.418×105 kJ/kg
Previous Answers
Submit
All attempts used; correct answer displayed
The enthalpy of formation of water can be used to calculate the amount of energy released per kilogram of fuel. Since only water is present in a nonstandard state, the enthalpy for the reaction is equal to the enthalpy of formation of
water. Thus
1 mol
0.002016 kg
AH H20 (kJ/mol) ×
where 0.002016 kg/mol
is the molar mass of hydrogen gas.
• Part C
Enter a balanced reaction for the complete combustion of methanol.
Express your answer as a chemical equation. Identify all of the phases in your answer.
2CH3OH(1)+ 302(g}>2CO2(g)+ 4H20(g)
Submit
Previous Answers
v Correct
The balanced combustion equation is
2CH3OH(1) + 302(g) → 2CO2(g) + 4H20(g)
![, Part D
Use standard enthalpies of formation to calculate the amount of heat released per kilogram of methanol fuel.
Express your answer using four significant figures.
amount of heat released = 2,267x104 kJ/kg
Submit
Previous Answers
v Correct
The enthalpies of formation of carbon dioxide, water, and methanol can be used to calculate the amount of energy released per kilogram of fuel as follows:
AHfxn =
npA Hf(products) – nrA Hf(reactants)
[(AH? CO2 + 2(A HỆ H20 ) – AH? CH3OH] (kJ/mol)
Once you have the enthalpy change for the reaction, divide by the molar mass of the fuel, methanol, in kg/mol
to find the heat released.
, Part E
Which fuel contains the most energy in the least mass?
hydrogen
methanol
Previous Answers
Submit
v Correct
Using the results from Parts B and D of the question. Hydrogen contributes almost an order of magnitude more energy per kilogram of mass than does methanol.
• Part F
To compare the energy of these fuels to that of octane (CgH19 , calculate the energy released per kilogram of octane.
Express your answer using four significant figures.
kJ/kg
amount of heat released by octane =](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F6a2c8a2e-5e75-47ce-a626-9c050ade62de%2F0621085b-de52-429c-8ca4-bff804be04ed%2Fiylqb6j.png&w=3840&q=75)
Transcribed Image Text:, Part D
Use standard enthalpies of formation to calculate the amount of heat released per kilogram of methanol fuel.
Express your answer using four significant figures.
amount of heat released = 2,267x104 kJ/kg
Submit
Previous Answers
v Correct
The enthalpies of formation of carbon dioxide, water, and methanol can be used to calculate the amount of energy released per kilogram of fuel as follows:
AHfxn =
npA Hf(products) – nrA Hf(reactants)
[(AH? CO2 + 2(A HỆ H20 ) – AH? CH3OH] (kJ/mol)
Once you have the enthalpy change for the reaction, divide by the molar mass of the fuel, methanol, in kg/mol
to find the heat released.
, Part E
Which fuel contains the most energy in the least mass?
hydrogen
methanol
Previous Answers
Submit
v Correct
Using the results from Parts B and D of the question. Hydrogen contributes almost an order of magnitude more energy per kilogram of mass than does methanol.
• Part F
To compare the energy of these fuels to that of octane (CgH19 , calculate the energy released per kilogram of octane.
Express your answer using four significant figures.
kJ/kg
amount of heat released by octane =
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning