Part A Indicate what type(s) of intermolecular forces are disrupted by the following denaturing treatments: Drag the appropriate Items to their respective bins. Reset Help egg whites whipped in a mixing bowl to make meringue an egg placed in water at 100 C and boiled for 10 minutes acid added to milk during the preparation of cheese Nonpolar attractions Salt bridges Hydrogen bonds Peptide bonds
Part A Indicate what type(s) of intermolecular forces are disrupted by the following denaturing treatments: Drag the appropriate Items to their respective bins. Reset Help egg whites whipped in a mixing bowl to make meringue an egg placed in water at 100 C and boiled for 10 minutes acid added to milk during the preparation of cheese Nonpolar attractions Salt bridges Hydrogen bonds Peptide bonds
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter14: Liquids And Solids
Section: Chapter Questions
Problem 93CP: Which of the following compound(s) exhibit only London dispersion intermolecular forces? Which...
Related questions
Question
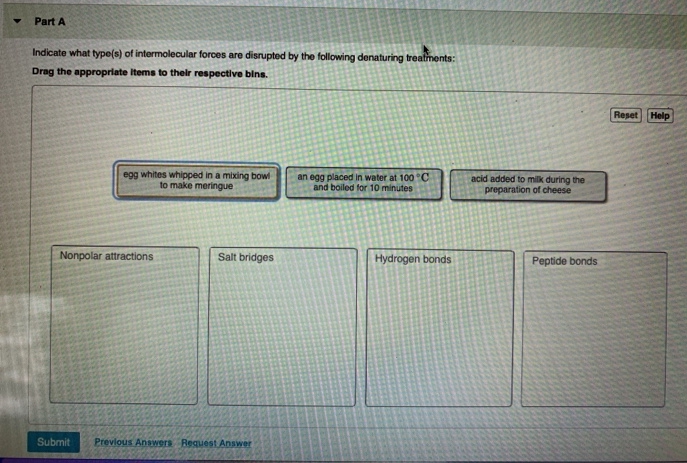
Transcribed Image Text:Part A
Indicate what type(s) of intermolecular forces are disrupted by the following denaturing treatments:
Drag the appropriate items to thelr respective bins.
Reset Help
egg whites whipped in a mixing bowl
to make meringue
an egg placed in water at 100 °C
and boiled for 10 minutes
acid added to milk during the
preparation of cheese
Nonpolar attractions
Salt bridges
Hydrogen bonds
Peptide bonds
Submit
Previous Answers Request Answer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning