Pavia, Lampman, Kriz, Engel escale Approach to Organric Laboratory Techniques 5/e this textbook require either of these techniques. If a filtration or decolorizing step is ever required, Technique 11 describes these procedures in detail. Pre-Lab Calculations 1. Calculate how much 95% ethyl alcohol will be required to dissolve 0.3 g of sulfa- nilamide at 78 C. Use the data for the graph in Technique 11, Figure 11.2 to make this calculation. The reason for making this calculation is so that you will know ahead of time the approximate amount of hot solvent you will be adding. 2. Using the volume of solvent calculated in Step 1, calculate how much sulfanilamide will remain dissolved in the mother liquor after the mixture is cooled to 0 C To dissolve the sulfanilamide in the minimum of hot (boiling or almost boiling) solvent, you must keep the mixture at (or near) the boiling point of 95%% ethyl alco- hol during the entire procedure. You wil-likely add more solvent than the amount you calculated because some solvent win evaporate. Ihe amount of solvent is cal- culated only to indicate the approximate amount of solvent required. You should follow the procedure to determine the correct amount.of solvent needed. CEDURE Preparations Weigh 0.30 Page 48 1,042 uc randh this solid to a 10-mL Erlenmeyer lask. Notente cooOneaPurest Tarnacrel To a second Erlenmever flask, a E hp 144 V 697 Crystallization: Purification of Solids TECHNIQUE 11 C (Poor solvent) Very soluble at all temperatures A (Good solvent) Very soluble at elevated temperatures Sparingly soluble at room temperatu re B (Poor solvent) Temperature Figure 11.1 Graph of solubility vs. temperature. at the boiling point of the solvent selected. The as can be seen 250 solubility curve should be steep, in line A of Figure 11.1. A curve with a low slope significant crystalliza- tion when the temperature of the solution was lowered. A solvent in which the material is very soluble at all temperatures (line C) also would not be a suitable crystallization solvent. The basic problem in performing a crystallization is to select a solvent (or mixed solvent) that provides a steep solubility-vs.- temperature curve for the material to be crystallized. A solvent that allows the behav- ior shown in line A is an ideal crystallization sol- vent. It should also be mentioned that solubility 200 (line B) would not cause 150 100 50 60° 80 40° 20° Temperature (C) curves are not always linear, they depicted in Figure 11.1. This figure represents an idealized form of solubility behavior. The solubility for sulfanilamide in 95% ethyl alcohol, shown in Figure 11.2, is typical of many organic compounds and shows what solubility behav- ior might look like for a real substance. This graph is based on the data in the follow- as are Figure 11.2 Solubility of sulfanilamide in 95% ethyl alcohol. curve ing table: Solubility (mg/mL) Temperature 0°C 14 20°C 24 40°C 46 60°C 88 80°C 210 The solubility of organic compounds is a function of the polarities of both the solvent and the solute (dissolved material). A general rule is "Like dissolves like." If the solute is very polar, a very polar solvent is needed to dissolve it; if the solute is nonpolar, a nonpolar solvent is needed. Applications of this rule are discussec extensively in Technique 10, Section 10.2, and in Section 11.5. canned, copied Solubility (mg/mL) Grams soluble-
Pavia, Lampman, Kriz, Engel escale Approach to Organric Laboratory Techniques 5/e this textbook require either of these techniques. If a filtration or decolorizing step is ever required, Technique 11 describes these procedures in detail. Pre-Lab Calculations 1. Calculate how much 95% ethyl alcohol will be required to dissolve 0.3 g of sulfa- nilamide at 78 C. Use the data for the graph in Technique 11, Figure 11.2 to make this calculation. The reason for making this calculation is so that you will know ahead of time the approximate amount of hot solvent you will be adding. 2. Using the volume of solvent calculated in Step 1, calculate how much sulfanilamide will remain dissolved in the mother liquor after the mixture is cooled to 0 C To dissolve the sulfanilamide in the minimum of hot (boiling or almost boiling) solvent, you must keep the mixture at (or near) the boiling point of 95%% ethyl alco- hol during the entire procedure. You wil-likely add more solvent than the amount you calculated because some solvent win evaporate. Ihe amount of solvent is cal- culated only to indicate the approximate amount of solvent required. You should follow the procedure to determine the correct amount.of solvent needed. CEDURE Preparations Weigh 0.30 Page 48 1,042 uc randh this solid to a 10-mL Erlenmeyer lask. Notente cooOneaPurest Tarnacrel To a second Erlenmever flask, a E hp 144 V 697 Crystallization: Purification of Solids TECHNIQUE 11 C (Poor solvent) Very soluble at all temperatures A (Good solvent) Very soluble at elevated temperatures Sparingly soluble at room temperatu re B (Poor solvent) Temperature Figure 11.1 Graph of solubility vs. temperature. at the boiling point of the solvent selected. The as can be seen 250 solubility curve should be steep, in line A of Figure 11.1. A curve with a low slope significant crystalliza- tion when the temperature of the solution was lowered. A solvent in which the material is very soluble at all temperatures (line C) also would not be a suitable crystallization solvent. The basic problem in performing a crystallization is to select a solvent (or mixed solvent) that provides a steep solubility-vs.- temperature curve for the material to be crystallized. A solvent that allows the behav- ior shown in line A is an ideal crystallization sol- vent. It should also be mentioned that solubility 200 (line B) would not cause 150 100 50 60° 80 40° 20° Temperature (C) curves are not always linear, they depicted in Figure 11.1. This figure represents an idealized form of solubility behavior. The solubility for sulfanilamide in 95% ethyl alcohol, shown in Figure 11.2, is typical of many organic compounds and shows what solubility behav- ior might look like for a real substance. This graph is based on the data in the follow- as are Figure 11.2 Solubility of sulfanilamide in 95% ethyl alcohol. curve ing table: Solubility (mg/mL) Temperature 0°C 14 20°C 24 40°C 46 60°C 88 80°C 210 The solubility of organic compounds is a function of the polarities of both the solvent and the solute (dissolved material). A general rule is "Like dissolves like." If the solute is very polar, a very polar solvent is needed to dissolve it; if the solute is nonpolar, a nonpolar solvent is needed. Applications of this rule are discussec extensively in Technique 10, Section 10.2, and in Section 11.5. canned, copied Solubility (mg/mL) Grams soluble-
Chapter81: Extractions, Separations, And Drying Agents
Section: Chapter Questions
Problem 5P
Related questions
Question
Prelab question

Transcribed Image Text:Pavia, Lampman, Kriz, Engel
escale Approach to Organric Laboratory Techniques 5/e
this textbook require either of these techniques. If a filtration or decolorizing step is
ever required, Technique 11 describes these procedures in detail.
Pre-Lab Calculations
1. Calculate how much 95% ethyl alcohol will be required to dissolve 0.3 g of sulfa-
nilamide at 78 C. Use the data for the graph in Technique 11, Figure 11.2 to make
this calculation. The reason for making this calculation is so that you will know
ahead of time the approximate amount of hot solvent you will be adding.
2. Using the volume of solvent calculated in Step 1, calculate how much sulfanilamide
will remain dissolved in the mother liquor after the mixture is cooled to 0 C
To dissolve the sulfanilamide in the minimum of hot (boiling or almost boiling)
solvent, you must keep the mixture at (or near) the boiling point of 95%% ethyl alco-
hol during the entire procedure. You wil-likely add more solvent than the amount
you calculated because some solvent win evaporate. Ihe amount of solvent is cal-
culated only to indicate the approximate amount of solvent required. You should
follow the procedure to determine the correct amount.of solvent needed.
CEDURE
Preparations
Weigh 0.30 Page 48 1,042 uc
randh this solid to a 10-mL Erlenmeyer
lask. Notente cooOneaPurest
Tarnacrel To a second Erlenmever flask,
a
E
hp
144
Transcribed Image Text:V
697
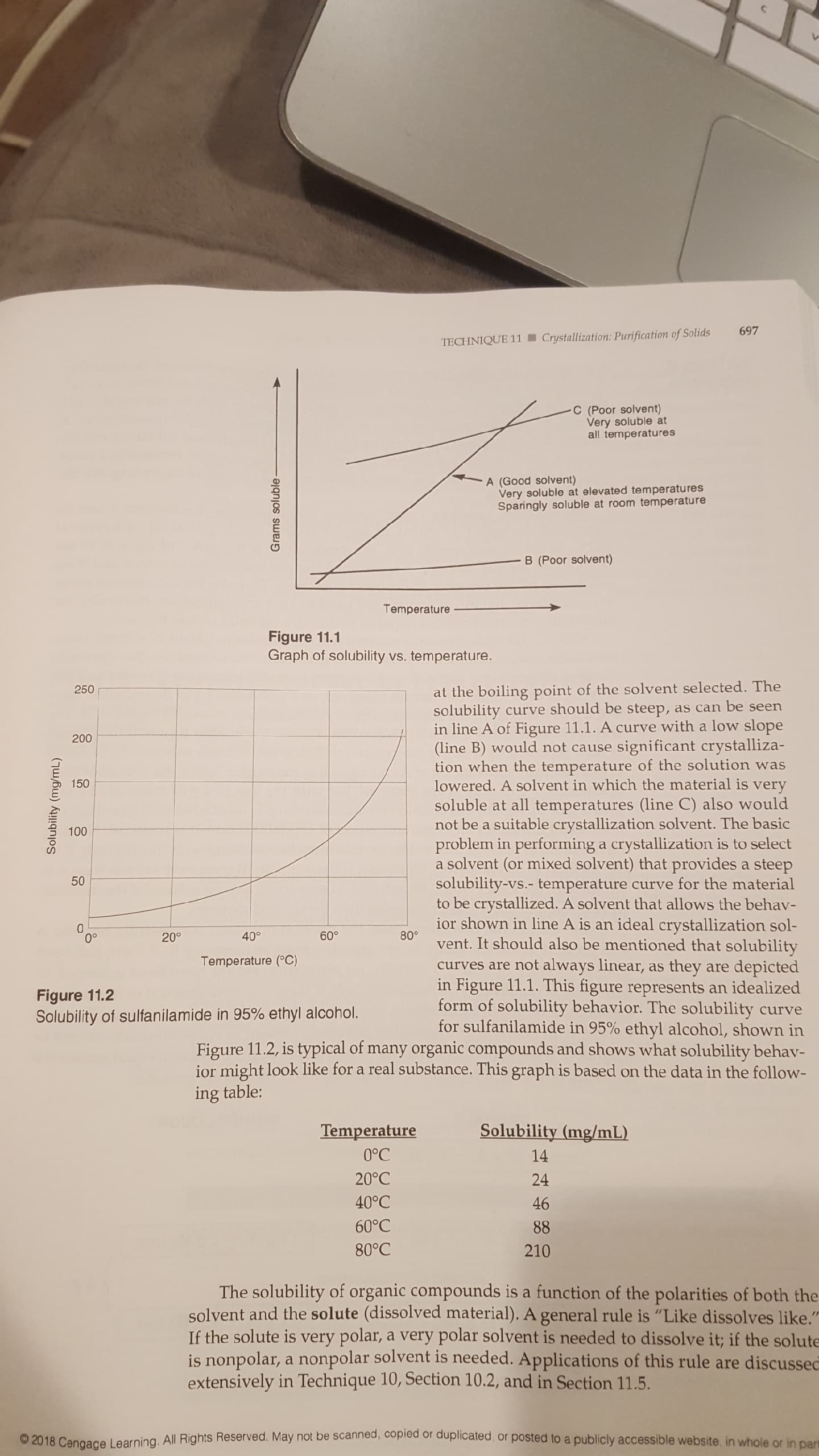
Crystallization: Purification of Solids
TECHNIQUE 11
C (Poor solvent)
Very soluble at
all temperatures
A (Good solvent)
Very soluble at elevated temperatures
Sparingly soluble at room temperatu re
B (Poor solvent)
Temperature
Figure 11.1
Graph of solubility vs. temperature.
at the boiling point of the solvent selected. The
as can be seen
250
solubility curve should be steep,
in line A of Figure 11.1. A curve with a low slope
significant crystalliza-
tion when the temperature of the solution was
lowered. A solvent in which the material is very
soluble at all temperatures (line C) also would
not be a suitable crystallization solvent. The basic
problem in performing a crystallization is to select
a solvent (or mixed solvent) that provides a steep
solubility-vs.- temperature curve for the material
to be crystallized. A solvent that allows the behav-
ior shown in line A is an ideal crystallization sol-
vent. It should also be mentioned that solubility
200
(line B) would not cause
150
100
50
60°
80
40°
20°
Temperature (C)
curves are not always linear,
they
depicted
in Figure 11.1. This figure represents an idealized
form of solubility behavior. The solubility
for sulfanilamide in 95% ethyl alcohol, shown in
Figure 11.2, is typical of many organic compounds and shows what solubility behav-
ior might look like for a real substance. This graph is based on the data in the follow-
as
are
Figure 11.2
Solubility of sulfanilamide in 95% ethyl alcohol.
curve
ing table:
Solubility (mg/mL)
Temperature
0°C
14
20°C
24
40°C
46
60°C
88
80°C
210
The solubility of organic compounds is a function of the polarities of both the
solvent and the solute (dissolved material). A general rule is "Like dissolves like."
If the solute is very polar, a very polar solvent is needed to dissolve it; if the solute
is nonpolar, a nonpolar solvent is needed. Applications of this rule are discussec
extensively in Technique 10, Section 10.2, and in Section 11.5.
canned, copied
Solubility (mg/mL)
Grams soluble-
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT