(PExercises: Molecules and Comp X PExercises: Thermochemistry Files X (218) Entha n.com/eplayer/book/2E5IYU4ZQG2/page/adf65754b38ca13018768451b8d38a08c17019f2b?platforms.id-mastering&b=2E5 Exercises: Thermochemistry If a bottle of nail polish remover contains 177 mL of acetone, how much heat is releas by its complete combustion? The density of acetone is 0.788 g/mL. MISSED THIS? Read Section 7.6; Watch IWE 7.7D 60. What mass of natural gas (CH) must burn to emit 267 kJ of heat CH4(g) +2 O2(g) CO2(g) + 2 H2O(g) AH 802.3 kJ Nitromethane (CH3NO2) burns in air to produce significant amounts of heat. 61. 2 CH3NO2 (1) 3/2 O2 (g)2 CO2 (g) +3 H20(1) + N2(g) AH -1418 kJ IXn How much heat is produced by the complete reaction of 5.56 kg of nitromethane? MISSED THIS? Read Section 7.6 Watch IWE 7.7 62. Titanium reacts with iodine to form titanium(III) iodide, emitting heat. 839 kJ 2 Ti(s) +3 I2 (g)2 Til3 (s) AH flc IXn Is it Ti Don'tr Determine the masses of titanium and iodine that react if 1.55 x 10 kJ of heat by the reaction. Invest The propane fuel (C3H3) used in gas barbeques burns according to the thermo 63. CLICK equation:
(PExercises: Molecules and Comp X PExercises: Thermochemistry Files X (218) Entha n.com/eplayer/book/2E5IYU4ZQG2/page/adf65754b38ca13018768451b8d38a08c17019f2b?platforms.id-mastering&b=2E5 Exercises: Thermochemistry If a bottle of nail polish remover contains 177 mL of acetone, how much heat is releas by its complete combustion? The density of acetone is 0.788 g/mL. MISSED THIS? Read Section 7.6; Watch IWE 7.7D 60. What mass of natural gas (CH) must burn to emit 267 kJ of heat CH4(g) +2 O2(g) CO2(g) + 2 H2O(g) AH 802.3 kJ Nitromethane (CH3NO2) burns in air to produce significant amounts of heat. 61. 2 CH3NO2 (1) 3/2 O2 (g)2 CO2 (g) +3 H20(1) + N2(g) AH -1418 kJ IXn How much heat is produced by the complete reaction of 5.56 kg of nitromethane? MISSED THIS? Read Section 7.6 Watch IWE 7.7 62. Titanium reacts with iodine to form titanium(III) iodide, emitting heat. 839 kJ 2 Ti(s) +3 I2 (g)2 Til3 (s) AH flc IXn Is it Ti Don'tr Determine the masses of titanium and iodine that react if 1.55 x 10 kJ of heat by the reaction. Invest The propane fuel (C3H3) used in gas barbeques burns according to the thermo 63. CLICK equation:
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter8: Thermochemistry
Section: Chapter Questions
Problem 12QAP: The heat of neutralization, Hneut, can be defined as the amount of heat released (or absorbed), q,...
Related questions
Question
Looking at #61. I got the correct answer of 6.46x10^4, but I don't understand why the solution is positive instead of negative.
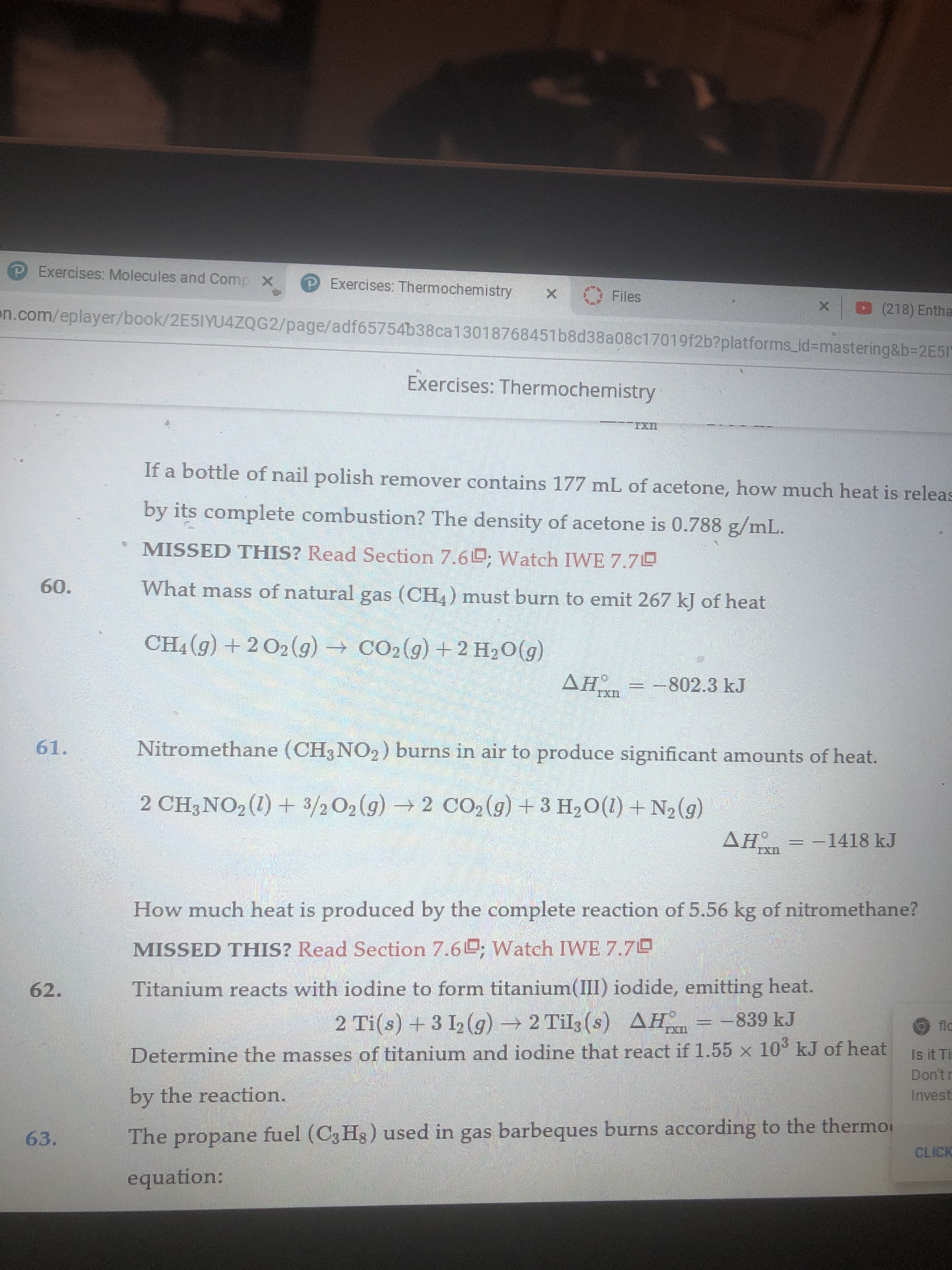
Transcribed Image Text:(PExercises: Molecules and Comp X PExercises: Thermochemistry
Files
X
(218) Entha
n.com/eplayer/book/2E5IYU4ZQG2/page/adf65754b38ca13018768451b8d38a08c17019f2b?platforms.id-mastering&b=2E5
Exercises: Thermochemistry
If a bottle of nail polish remover contains 177 mL of acetone, how much heat is releas
by its complete combustion? The density of acetone is 0.788 g/mL.
MISSED THIS? Read Section 7.6; Watch IWE 7.7D
60.
What mass of natural gas (CH) must burn to emit 267 kJ of heat
CH4(g) +2 O2(g)
CO2(g) + 2 H2O(g)
AH 802.3 kJ
Nitromethane (CH3NO2) burns in air to produce significant amounts of heat.
61.
2 CH3NO2 (1) 3/2 O2 (g)2 CO2 (g) +3 H20(1) + N2(g)
AH
-1418 kJ
IXn
How much heat is produced by the complete reaction of 5.56 kg of nitromethane?
MISSED THIS? Read Section 7.6
Watch IWE 7.7
62.
Titanium reacts with iodine to form titanium(III) iodide, emitting heat.
839 kJ
2 Ti(s) +3 I2 (g)2 Til3 (s) AH
flc
IXn
Is it Ti
Don'tr
Determine the masses of titanium and iodine that react if 1.55 x 10 kJ of heat
by the reaction.
Invest
The propane fuel (C3H3) used in gas barbeques burns according to the thermo
63.
CLICK
equation:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning