Ph (s. 25°C, 1 atm) Ph (s. 42.5°C, 1 atm) Ph (1, 42.5°C, 1 atm) Ph (1,181.4°C, 1 atm) Ph=phenol(C,H,OH) (True path) AR, AR₂ AR, ارد AR₂ Ph (v. 300.0°C, 3 atm) AR Ph (v. 300.0°C, 1 atm) AR₂ Ph (v. 181.4°C, 1 atm)
Ph (s. 25°C, 1 atm) Ph (s. 42.5°C, 1 atm) Ph (1, 42.5°C, 1 atm) Ph (1,181.4°C, 1 atm) Ph=phenol(C,H,OH) (True path) AR, AR₂ AR, ارد AR₂ Ph (v. 300.0°C, 3 atm) AR Ph (v. 300.0°C, 1 atm) AR₂ Ph (v. 181.4°C, 1 atm)
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
For the solid, liquid, and vapor phases of phenol, follow the hypothetical process path shown. Calculate the each enthalpy. Use the formula DeltaH = VdeltaP. Also use Table B.1 for the phenol properties in the Elementary Principles of Chemical Processes. The problem begins where it is underlined in blue.
Transcribed Image Text:||!
PDF *Elementary Principles of Chemic X +
60°F
Clear
File | E:/Elementary%20Principles%20of%20Chemical%20Processes,%204th%20Edition%20(%20PDF Drive%20).pdf
Draw
2 T Read aloud
406 CHAPTER 8 Balances on Nonreactive Processes
Test Yourself
426 of 695
OD
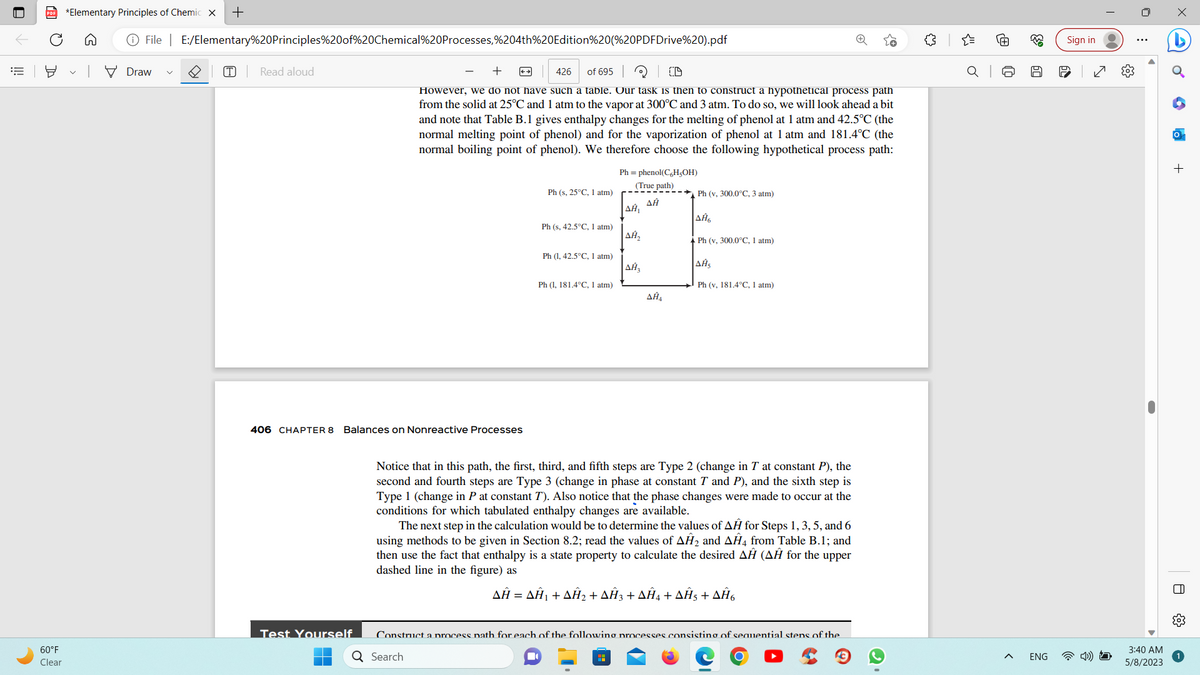
However, we do not have such a table. Our task is then to construct a hypothetical process path
from the solid at 25°C and 1 atm to the vapor at 300°C and 3 atm. To do so, we will look ahead a bit
and note that Table B.1 gives enthalpy changes for the melting of phenol at 1 atm and 42.5°C (the
normal melting point of phenol) and for the vaporization of phenol at 1 atm and 181.4°C (the
normal boiling point of phenol). We therefore choose the following hypothetical process path:
Ph (s, 25°C, 1 atm)
Q Search
Ph (s, 42.5°C, 1 atm)
Ph (1, 42.5°C, 1 atm)
Ph (1, 181.4°C, 1 atm)
Ph=phenol(C,H,OH)
(True path)
AĤ
AĤ₁
ΔΗ͂,
AĤ3
ΔΗ͂Α
Ph (v. 300.0°C, 3 atm)
AĤ6
Ph (v, 300.0°C, 1 atm)
Δῆς
Ph (v, 181.4°C, 1 atm)
Notice that in this path, the first, third, and fifth steps are Type 2 (change in T at constant P), the
second and fourth steps are Type 3 (change in phase at constant T and P), and the sixth step is
Type 1 (change in P at constant T). Also notice that the phase changes were made to occur at the
conditions for which tabulated enthalpy changes are available.
The next step in the calculation would be to determine the values of AĤ for Steps 1, 3, 5, and 6
using methods to be given in Section 8.2; read the values of AH₂ and AĤ4 from Table B.1; and
then use the fact that enthalpy is a state property to calculate the desired AĤ (AĦ for the upper
dashed line in the figure) as
AĤ = AĤ₁ + AĤ₂ + AĤ3 + AĤ4 + AĤ5 + AĤ6
Construct a process nath for each of the following processes consisting of sequential stens of the
{"
J
63
50
^ ENG
Sign in
I
00
3:40 AM
5/8/2023
+
☐
{0}
Transcribed Image Text:X +
PDF *Elementary Principles of Chemic X b My Questions | bartleby
File | E:/Elementary%20Principles%20of%20Chemical%20Processes,%204th%20Edition%20(%20PDF Drive%20).pdf
Draw
(T)
Read aloud
of 695 3
its mole fraction in the mixture). A state property does not depend on how the species reached its
state. Consequently, when a species passes from one state to another, both ▲Û and ▲H for the
process are independent of the path taken from the first state to the second one.
←→
425
In most of this chapten and in Chapter 9, you will learn how to calculate internal energy and
enthalpy changes associated with certain processes: specifically,
Changes in P at constant T and state of aggregation (Section 8.2).
2. Changes in T at constant P and state of aggregation (Section 8.3).
3. Phase changes at constant T and P-melting, solidifying, vaporizing, condensing, sublimating
(Section 8.4).
4. Mixing of two liquids or dissolving of a gas or a solid in a liquid at constant T and P (Section 8.5).
5. Chemical reaction at constant T and P (Chapter 9).
Q Search
For example, compressing hydrogen gas from 1 atm to 300 atm at 25°C is a Type 1 process;
melting ice at 0°C and then heating the liquid water to 30°C, all at 1 atm, is a Type 3 process
followed by a Type 2 process; mixing sulfuric acid and water at a constant temperature of 20°C
and a constant pressure of 1 atm is a Type 4 process.
Once we know how to calculate AU and AH for these five types of processes, we can
calculate these quantities for any process by taking advantage of the fact that Û and Ĥ are state
properties. The procedure is to construct a hypothetical process path from the initial state to the
final state consisting of a series of steps of the given five types. Having done this, we calculate AĤ
for each of the steps, and then add the AA's for the steps to calculate AĤ for the total process.
Since Ĥ is a state property, AĤ calculated for the hypothetical process path-which we
constructed for convenience is the same as AĤ for the path actually followed by the process.
The same procedure can be followed to calculate AU for any process.
Suppose, for example, that we wish to calculate AĤ for a process in which solid phenol at
25°C and I atm is converted to phenol vapor at 300°C and 3 atm. If we had a table of enthalpies for
phenol, we could simply subtract Ĥ at the initial state from Ĥ at the final state, or
AĤ = Ĥ(vapor, 300°C, 3 atm) - Ĥ(solid, 25°C, 1 atm)
However, we do not have such a table. Our task is then to construct a hypothetical process path
from the solid at 25°C and 1 atm to the vapor at 300°C and 3 atm. To do so, we will look ahead a bit
and note that Table B.1 gives enthalpy changes for the melting of phenol at 1 atm and 42.5°C (the
normal melting point of phenol) and for the vaporization of phenol at 1 atm and 181.4°C (the
normal boiling point of phenol). We therefore choose the following hypothetical process path:
Phnhenol(C₂H-OH).
{"
J
63
50
ENG
Sign in
I
●
5:53 AM
5/8/2023
+
O
{0}
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 2 images

Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The