Phosphate buffer solution is purchased as a(n) 2x stock solution. The solution is more concentrated than the working stock. If tasked with making 200 mL of a 1x solution of PBS, how many mL of the stock solution would be needed? Report your answer in standard decimal notation rounded to one decimal place. How many microliters of original sample are required to produce a final dilution of 103 in a total volume of 20 mL? 1 microliter is 10-6 L or 103 mL. • The dilution is equal to M2/M1
Phosphate buffer solution is purchased as a(n) 2x stock solution. The solution is more concentrated than the working stock. If tasked with making 200 mL of a 1x solution of PBS, how many mL of the stock solution would be needed? Report your answer in standard decimal notation rounded to one decimal place. How many microliters of original sample are required to produce a final dilution of 103 in a total volume of 20 mL? 1 microliter is 10-6 L or 103 mL. • The dilution is equal to M2/M1
Chapter7: Solutions And Colloids
Section: Chapter Questions
Problem 7.39E
Related questions
Question
please highlight the correct answer
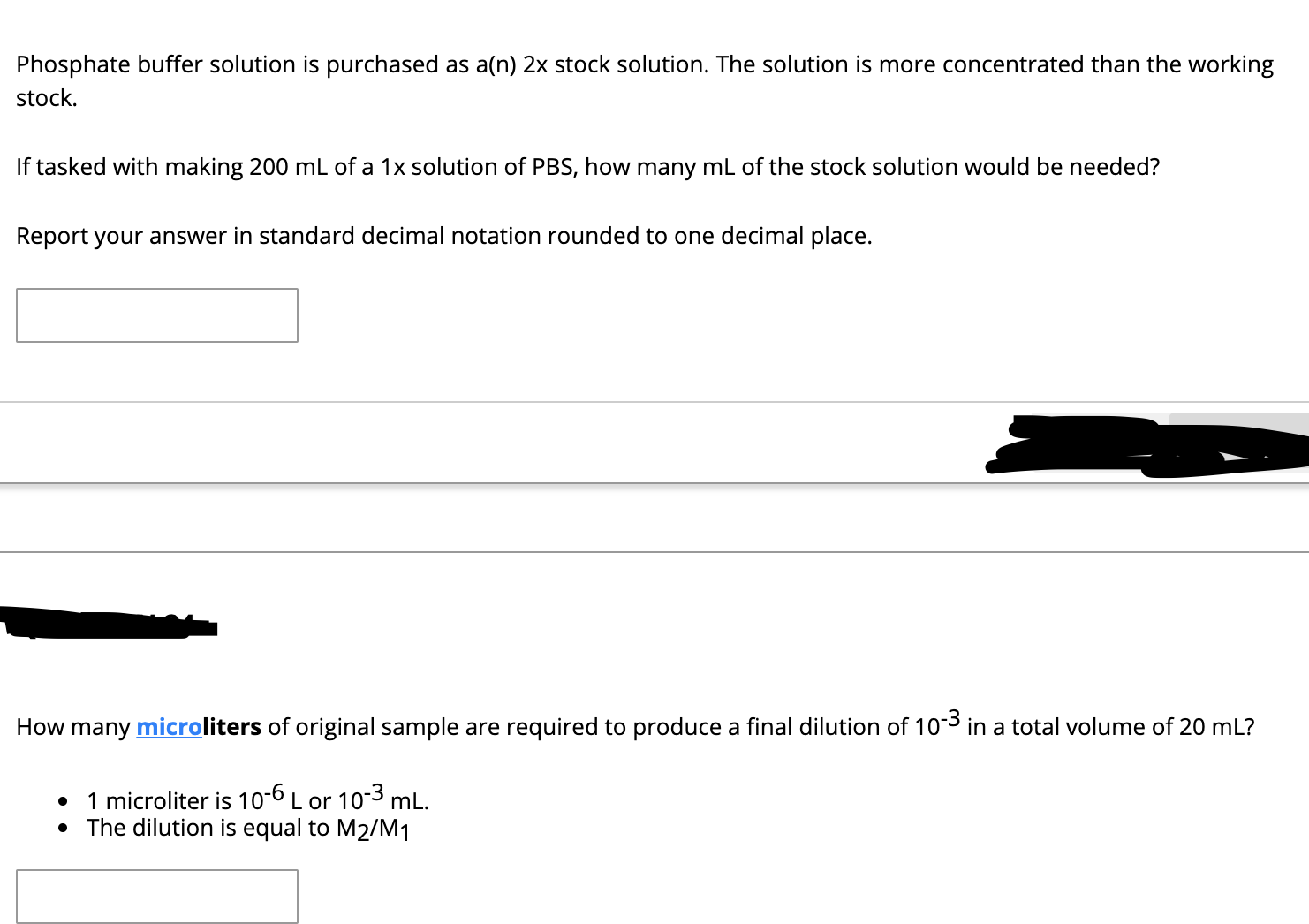
Transcribed Image Text:Phosphate buffer solution is purchased as a(n) 2x stock solution. The solution is more concentrated than the working
stock.
If tasked with making 200 mL of a 1x solution of PBS, how many mL of the stock solution would be needed?
Report your answer in standard decimal notation rounded to one decimal place.
How many microliters of original sample are required to produce a final dilution of 103 in a total volume of 20 mL?
1 microliter is 10-6 L or 103 mL.
• The dilution is equal to M2/M1
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning