Photoelectron spectroscopy studies of silicon atoms excited by X-rays with wavelength 9.890 × 10-1º m show four peaks in which the electrons have speeds 2.097 × 107 m s-!, 2.093 x 107 m s-!, 2.014 × 107 m s', and 1.971 x 107 m s-1. (Recall that 1 J = 1 kg m² s-2.) (a) Calculate the ionization energy of the electrons in each peak. (b) Assign each peak to an orbital of the silicon atom.
Photoelectron spectroscopy studies of silicon atoms excited by X-rays with wavelength 9.890 × 10-1º m show four peaks in which the electrons have speeds 2.097 × 107 m s-!, 2.093 x 107 m s-!, 2.014 × 107 m s', and 1.971 x 107 m s-1. (Recall that 1 J = 1 kg m² s-2.) (a) Calculate the ionization energy of the electrons in each peak. (b) Assign each peak to an orbital of the silicon atom.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter5: Quantum Mechanics And Atomic Structure
Section: Chapter Questions
Problem 32P
Related questions
Question
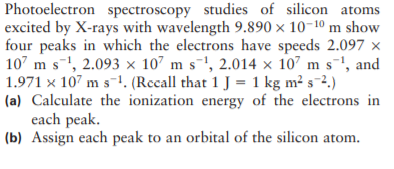
Transcribed Image Text:Photoelectron spectroscopy studies of silicon atoms
excited by X-rays with wavelength 9.890 × 10-1º m show
four peaks in which the electrons have speeds 2.097 ×
107 m s-!, 2.093 x 107 m s-!, 2.014 × 107 m s', and
1.971 x 107 m s-1. (Recall that 1 J = 1 kg m² s-2.)
(a) Calculate the ionization energy of the electrons in
each peak.
(b) Assign each peak to an orbital of the silicon atom.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 7 steps

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,