pis) Large quantities of alcohol have been contaminated due to a leak in the storage tank, which needs to be remediated by the process below. The contaminated alcohol contains large amounts of alcohol and ol in an aqueous (i.e., water) solution. A typical process first passes a certain amount of this solution through a settler to remove most of the soil in the form of pure solid soil, at a rate of 85 kg/hr (m2) from the settler tank. The remaining liquid of the settler enters a distillation column to separate the solution into 2 streams: 1) the top stream contains alcohol-rich solution with 99 wt% alcohol and 1 wt% water, and 2) the bottom stream contains water-rich solution with 1 wt% alcohol, 96 wt% water, and 3 wt% soil. Half of the water-rich solution eXiting from the bottom of the distillation column is recycled (m,) and mixed with the solid product of the setteler to form contaminant solution. If the flowrate of the top stream of the distillation column (m4) is 1000 kg/hr and flowrate of the contaminant solution after the mixing point (mg) is 100 kg/hr: December 14, 2019 a) Define your sub-systems and perform a DOF analysis on each sub-system. Clearly indicate the unknowns and equations for each sub-system. Hint: the composition of the streams involving the split point of the recycle stream is exactly the same, so there is only one species balance equation for this unit instead of 3. b) What is the mass flow rate of the recycle stream (m,) in kg/hr? 6. c) What is the composition (yA3, Yw3, Ys3) of the stream entering the distillation column (in mass fractions)? d) What should be the flowrate of the feed (m,) in kg/hr? та = 1000 kg/hr m3 Y44 = 0.99 Settler Distillation Yw4 = 0.01 УА1 УАЗ Уw1 Уw3 Ys1 Ys3 m2 = 85 kg/hr YA5 = 0.01 Yws = 0.96 Yss = 0.03 Ys2 = 1.0 %3D YA7 = 0.01 YA6 = 0.01 %3D Yw6 = 0.96 Ys6 = 0.03 УА8 ṁg = 100 kg/hr Yw7 = 0.96 %3D Уws Ys7 = 0.03 Ys8
pis) Large quantities of alcohol have been contaminated due to a leak in the storage tank, which needs to be remediated by the process below. The contaminated alcohol contains large amounts of alcohol and ol in an aqueous (i.e., water) solution. A typical process first passes a certain amount of this solution through a settler to remove most of the soil in the form of pure solid soil, at a rate of 85 kg/hr (m2) from the settler tank. The remaining liquid of the settler enters a distillation column to separate the solution into 2 streams: 1) the top stream contains alcohol-rich solution with 99 wt% alcohol and 1 wt% water, and 2) the bottom stream contains water-rich solution with 1 wt% alcohol, 96 wt% water, and 3 wt% soil. Half of the water-rich solution eXiting from the bottom of the distillation column is recycled (m,) and mixed with the solid product of the setteler to form contaminant solution. If the flowrate of the top stream of the distillation column (m4) is 1000 kg/hr and flowrate of the contaminant solution after the mixing point (mg) is 100 kg/hr: December 14, 2019 a) Define your sub-systems and perform a DOF analysis on each sub-system. Clearly indicate the unknowns and equations for each sub-system. Hint: the composition of the streams involving the split point of the recycle stream is exactly the same, so there is only one species balance equation for this unit instead of 3. b) What is the mass flow rate of the recycle stream (m,) in kg/hr? 6. c) What is the composition (yA3, Yw3, Ys3) of the stream entering the distillation column (in mass fractions)? d) What should be the flowrate of the feed (m,) in kg/hr? та = 1000 kg/hr m3 Y44 = 0.99 Settler Distillation Yw4 = 0.01 УА1 УАЗ Уw1 Уw3 Ys1 Ys3 m2 = 85 kg/hr YA5 = 0.01 Yws = 0.96 Yss = 0.03 Ys2 = 1.0 %3D YA7 = 0.01 YA6 = 0.01 %3D Yw6 = 0.96 Ys6 = 0.03 УА8 ṁg = 100 kg/hr Yw7 = 0.96 %3D Уws Ys7 = 0.03 Ys8
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
100%
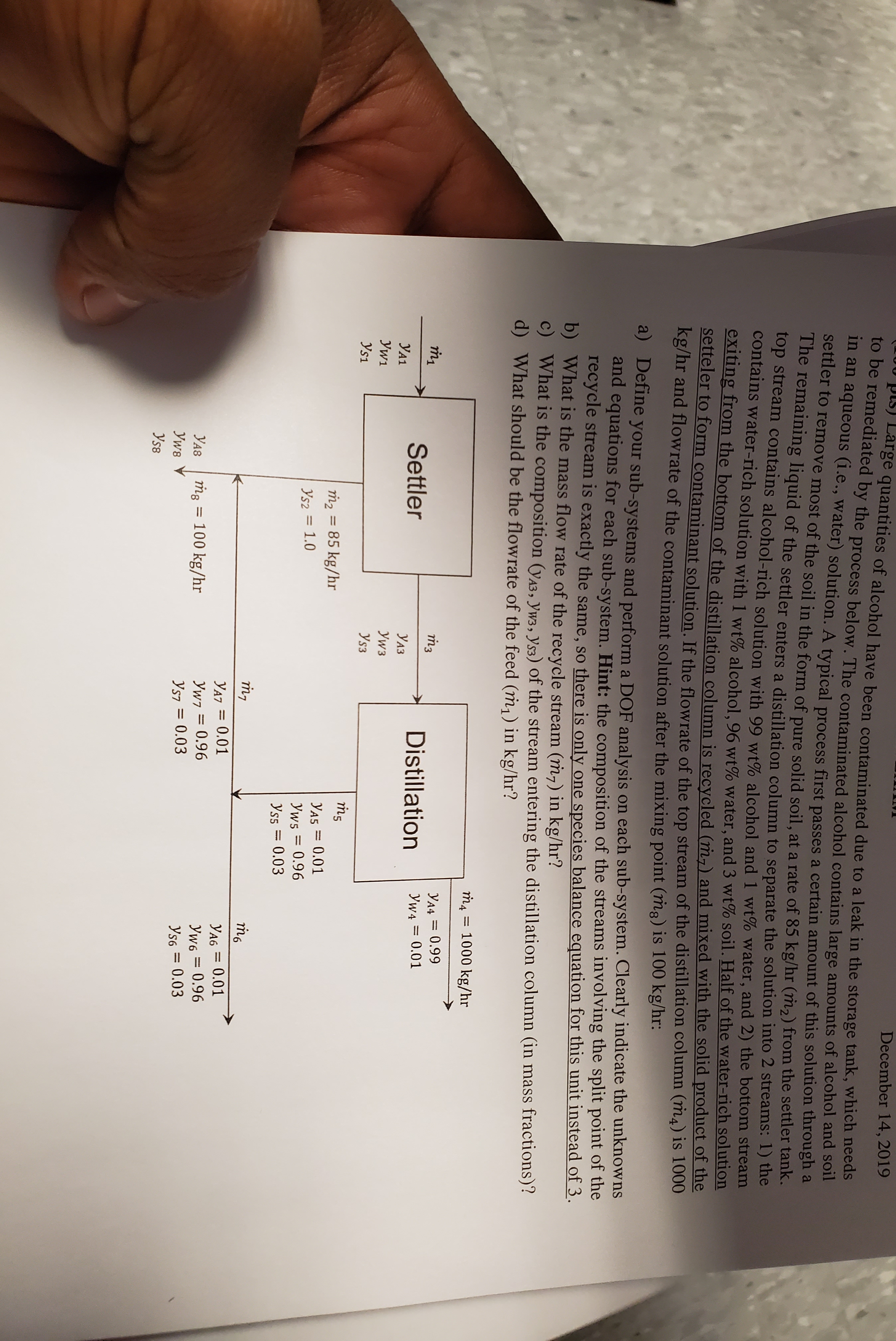
Transcribed Image Text:pis) Large quantities of alcohol have been contaminated due to a leak in the storage tank, which needs
to be remediated by the process below. The contaminated alcohol contains large amounts of alcohol and ol
in an aqueous (i.e., water) solution. A typical process first passes a certain amount of this solution through a
settler to remove most of the soil in the form of pure solid soil, at a rate of 85 kg/hr (m2) from the settler tank.
The remaining liquid of the settler enters a distillation column to separate the solution into 2 streams: 1) the
top stream contains alcohol-rich solution with 99 wt% alcohol and 1 wt% water, and 2) the bottom stream
contains water-rich solution with 1 wt% alcohol, 96 wt% water, and 3 wt% soil. Half of the water-rich solution
eXiting from the bottom of the distillation column is recycled (m,) and mixed with the solid product of the
setteler to form contaminant solution. If the flowrate of the top stream of the distillation column (m4) is 1000
kg/hr and flowrate of the contaminant solution after the mixing point (mg) is 100 kg/hr:
December 14, 2019
a) Define your sub-systems and perform a DOF analysis on each sub-system. Clearly indicate the unknowns
and equations for each sub-system. Hint: the composition of the streams involving the split point of the
recycle stream is exactly the same, so there is only one species balance equation for this unit instead of 3.
b) What is the mass flow rate of the recycle stream (m,) in kg/hr?
6.
c) What is the composition (yA3, Yw3, Ys3) of the stream entering the distillation column (in mass fractions)?
d) What should be the flowrate of the feed (m,) in kg/hr?
та
= 1000 kg/hr
m3
Y44 = 0.99
Settler
Distillation
Yw4 = 0.01
УА1
УАЗ
Уw1
Уw3
Ys1
Ys3
m2 = 85 kg/hr
YA5 = 0.01
Yws = 0.96
Yss = 0.03
Ys2 = 1.0
%3D
YA7 = 0.01
YA6 = 0.01
%3D
Yw6 = 0.96
Ys6 = 0.03
УА8
ṁg = 100 kg/hr
Yw7 = 0.96
%3D
Уws
Ys7 = 0.03
Ys8
Expert Solution
Trending now
This is a popular solution!
Step by step
Solved in 9 steps with 9 images

Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The